Phytochemistry
Phytochemistry is the study of plant-derived chemicals, also named - the phytochemicals.
Those who are researching phytochemistry seek to identify the structures of a large number of secondary metabolic compounds found in plants, the roles of these compounds in human and plant biology, and their biosynthesis.
For numerous factors, plants synthesize phytochemicals one example of which is to defend themselves from insect attacks and plant diseases.
Phytochemicals in food plants are often used in human biology and have health benefits in many cases. The substances found in plants are of various types, but most are in four main biochemical classes: alkaloids, glycosides, polyphenols, and terpenes.
The Phytochemistry of Cherokee Aromatic Medicinal Plants
William N. Setzer 1,2
1 Department of Chemistry, University of Alabama in Huntsville, Huntsville, AL 35899, USA;
wsetzer@chemistry.uah.edu; Tel.: +1-256-824-6519
2 Aromatic Plant Research Center, 230 N 1200 E, Suite 102, Lehi, UT 84043, USA
Received: 25 October 2018; Accepted: 8 November 2018; Published: 12 November 2018
Introduction
Natural products have been an important source of medicinal agents throughout history and
modern medicine continues to rely on traditional knowledge for treatment of human maladies [1].
Traditional medicines such as Traditional Chinese Medicine [2], Ayurvedic [3], and medicinal plants
from Latin America [4] have proven to be rich resources of biologically active compounds and potential
new drugs. Several plant-derived drugs are in use today, including, for example, vinblastine (from
Catharanthus roseus (L.) G. Don, used to treat childhood leukemia); paclitaxel (from Taxus brevifolia Nutt., used to treat ovarian cancer); morphine (from Papaver somniferum L., used to treat pain); and quinine (from Cinchona spp., used to treat malaria) [5]. Not only are phytochemicals useful medicines in their own right, but compounds derived from them or inspired by them have become useful medicines [6,7].
For example, Artemisia annua L., a plant originally used in Traditional Chinese Medicine to treat fever,
is the source of artemisinin, a clinically-useful antimalarial sesquiterpenoid [8]; the antihypertensive
drug reserpine, isolated from the roots of Rauvolfia serpentina (L.) Benth. ex Kurz., has been used
in Ayurveda to treat insanity, epilepsy, insomnia, hysteria, eclampsia, as well as hypertension [9];
Dysphania ambrosioides (L.) Mosyakin and Clemants (syn. Chenopodium ambrosioides L.) is used in several Latin American cultures as an internal anthelmintic and external antiparasitic [4] and has shown
promise for treatment of cutaneous leishmaniasis [10].
The biological activity of D. ambrosioides has been attributed to the monoterpenoid endoperoxide ascaridole.
Unfortunately, much of the traditional medicine knowledge of Native North American peoples
has been lost due to population decimation and displacement from their native lands by European
conquerors (see, for example: [11–14]). Nevertheless, there are still some remaining sources of information about Native American ethnobotany [15,16]. In addition, there are several sources of
Cherokee ethnobotany [17–22].
The Cherokee Native Americans are a tribe of Iroquoian-language people who lived in the
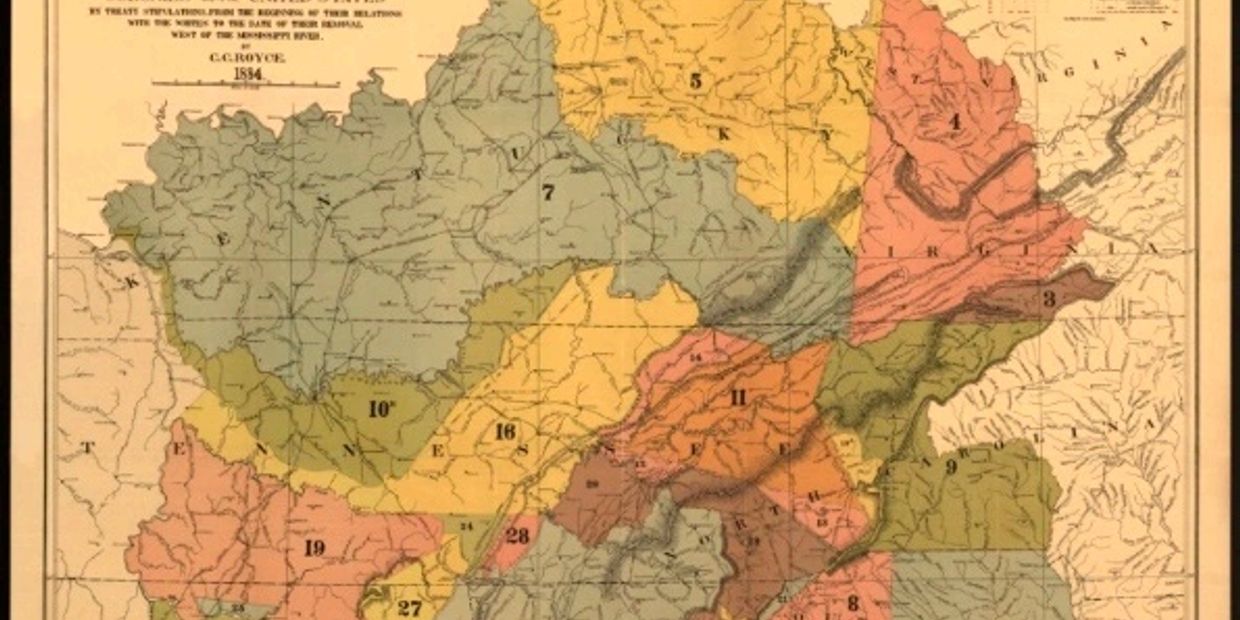
southern part of the Appalachian Mountain region in present-day northern Georgia, eastern Tennessee, and western North Carolina and South Carolina at the time of European contact [13] (Figure 1A).
During and after the American Revolution, Cherokee wars with European settlers resulted in the
surrender of vast amounts of territory. Gold was discovered on Cherokee land in north Georgia
and the Treaty of New Echota (1835) ceded all Cherokee land east of the Mississippi River to the
United States. Congress passed the Indian Removal Act in 1830, and the forced eviction of as many as
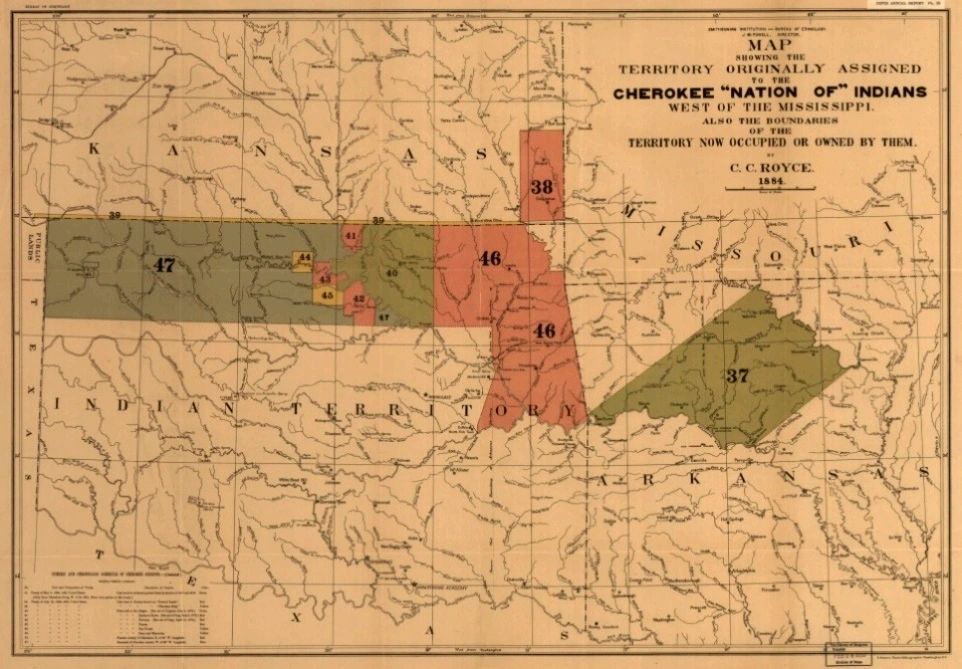
16,000 Cherokee took place during the fall and winter of 1838–1839 to a new territory in north-eastern Oklahoma (Fibure 1B). During this “Trail of Tears”, an estimated one-fourth of the Cherokee died.
However, at the time of the removal, a few hundred Cherokee successfully escaped to the mountains
of western North Carolina, forming what is now the Eastern Band of Cherokee Indians.
In this review, I have consulted the ethnobotanical sources for plants used in Cherokee
traditional medicine [15–24] and I have carried out a literature search using Google Scholar, PubMed,
ResearchGate, and Science Direct for phytochemical analyses on the plant species. Note that in many
instances, the phytochemistry was determined by plants not collected in the south-eastern United
States; many of the species have been introduced to other parts of the world and some species are native to other continents besides North America. The phytochemistry, therefore, may be affected by the different geographical and climatic conditions [25]. Sources reporting the phytochemical constituents, regardless of geographical origin, have been included.
Medicines 2018, 5, x FOR PEER REVIEW 2 of 90
Unfortunately, much of the traditional medicine knowledge of Native North American peoples
has been lost due to population decimation and displacement from their native lands by European
conquerors (see, for example: [11–14]). Nevertheless, there are still some remaining sources of
information about Native American ethnobotany [15,16]. In addition, there are several sources of
Cherokee ethnobotany [17–22].
The Cherokee Native Americans are a tribe of Iroquoian-language people who lived in the
southern part of the Appalachian Mountain region in present-day northern Georgia, eastern
Tennessee, and western North Carolina and South Carolina at the time of European contact [13]
(Figure 1A). During and after the American Revolution, Cherokee wars with European settlers
resulted in the surrender of vast amounts of territory. Gold was discovered on Cherokee land in north
Georgia and the Treaty of New Echota (1835) ceded all Cherokee land east of the Mississippi River to
the United States. Congress passed the Indian Removal Act in 1830, and the forced eviction of as many
as 16,000 Cherokee took place during the fall and winter of 1838–1839 to a new territory in north-eastern Oklahoma (Fibure 1B). During this “Trail of Tears”, an estimated one-fourth of the Cherokee died.
However, at the time of the removal, a few hundred Cherokee successfully escaped to the mountains
of western North Carolina, forming what is now the Eastern Band of Cherokee Indians.
In this review, I have consulted the ethnobotanical sources for plants used in Cherokee
traditional medicine [15–24] and I have carried out a literature search using Google Scholar, PubMed,
ResearchGate, and Science Direct for phytochemical analyses on the plant species. Note that in many
instances, the phytochemistry was determined by plants not collected in the south-eastern United
States; many of the species have been introduced to other parts of the world and some species are
native to other continents besides North America. The phytochemistry, therefore, may be affected by
the different geographical and climatic conditions [25]. Sources reporting the phytochemical
constituents, regardless of geographical origin, have been included
Figure 1.(A)
Cherokee territorial lands [26]. (A) ′′Map of the former territorial limits of the Cherokee ′Nation of′ Indians′′, i.e., prior to displacement of Euro-Americans.
Cherokee Aromatic Medicinal Plants and Their Phytochemical Constituents
The plants used by the Cherokee people for traditional medicines for which the phytochemistry
has been investigated are summarized in Table 1.
Figure 1.(B)
"Map showing the territory originally assigned Cherokee ′Nation of′ Indians′′, i.e., after the forcible relocation known as the ′′Trail of Tears”
Cherokee Aromatic Medicinal Plants in Use as Herbal Medicine
Achillea millefolium L
Achillea millefolium (yarrow) is native to temperate regions of the Northern Hemisphere but
has been introduced worldwide [510]. The traditional medical uses of A. millefolium have been
reviewed and the plant has been used since ancient times as a wound-healing agent and to treat
gastrointestinal complaints [510–512]. Consistent with this, the Cherokee have also used A. millefolium as an antihemorrhagic; for healing wounds, treating bloody hemorrhoids and bloody urine, and for bowel complaints [15,17,510]. In addition, infusions of A. millefolium have been used as a treatment for fever [15,17,510]. Yarrow extract has shown spasmogenic effects on murine and human gastric antrum, consistent with its traditional use to treat dyspepsia [513]. In a double-blind clinical trial, A. millefolium ointment was shown to reduce pain, inflammation, and ecchymosis in episiotomy wound healing [514].
The essential oils of A. millefolium have shown wide variation depending on geographical location
and growing season. Volatile oil samples from Turkey [48] and Macedonia [51] were dominated by
1,8-cineole and camphor, whereas the essential oil from Lavras, Brazil, was rich in chamazulene [49].
The essential oil from Lithuania showed wide variation in composition depending on morphological
type (flower color) as well as plant phenology [50]; γ-terpinene and cadinene (isomer not identified)
were the major components during the flowering phase, but β-pinene was abundant during the
vegetative phase. Conversely, A. millefolium leaf essential oil from Portugal was rich in 1,8-cineole
during the flowering phase, but germacrene D dominated the oil during the vegetative phase [53].
The non-volatile chemical components of A. millefolium are generally dominated by phenolics
(e.g., chlorogenic acid and other quinic acid derivatives) and flavonoids and flavonoid glycosides (e.g.,
luteolin, apigenin, and quercetin, and their glycosides) [38–42,44,46,47]. Chlorogenic acid has shown
in vivo wound-healing properties in rat models [515,516]. Likewise, the flavonoid apigenin [517,518]
as well as an apigenin glycoside [519] have shown in vivo wound-healing effects in rodent models.
Similarly, luteolin [520–522], luteolin-7-O-glucoside [523], quercetin [524–526] and several quercetin
glycosides [527–531] have shown wound-healing effects.
Caulophyllum thalictroides (L.) Michx.
A decoction of the roots of C. thalictroides (blue cohosh) has been used by the Cherokee as an
anticonvulsive (to treat “fits and hysterics”) and antirheumatic [15]. The plant is also used as a
gynecological aid, to promote childbirth and to treat womb inflammation [15].
These traditional uses are in apparent contrast to the observed toxic effects (convulsions, respiratory paralysis) of the plant observed in range animals such as sheep [108]. The rhizome of C. thalictroides contains several quinolizidine alkaloids, including N-methylcytisine (also known as caulophylline), baptifoline, anagyrine, and lupanine [108,110,112]. N-Methylcytisine is known to stimulate the central nervous system, and in high doses causes convulsions followed by paralysis [532]. Acute lupanine toxicity is characterized by neurotoxic effects including decreased cardiac contractility, blocking of ganglionic transmission and contraction of uterine smooth muscle [533].
This latter effect explains the traditional Cherokee use to promote childbirth. Apparently, lupanine, in lower doses, does not exhibit sub-chronic, chronic, reproductive, or mutagenic toxic effects [533].
Both N-methylcytisine [110] and anagyrine [534] have been shown to be teratogenic, however. The aporphine alkaloid magnoflorine, on the other hand, has shown sedative and anxiolytic effects [535] and may be responsible for the anti-convulsive and sedative uses of C. thalictroides in Cherokee traditional medicine.
Lee and co-workers [115] have shown that the oleanolic acid glycosides caulosides A–D exert
anti-inflammatory effects by way of inhibiting expression of inducible nitric oxide synthase (iNOS)
and the pro-inflammatory cytokines tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6).
The anti-inflammatory effects of C. thalictroides triterpene saponins are consistent with the Cherokee
traditional uses to treat rheumatism and inflammation.
Cimicifuga racemosa (L.) Nutt. (syn. Actaea racemosa L.)
Black cohosh (C. racemosa) has been a popular herbal supplement for many years [536]. The plant
is reputed to possess anti-inflammatory, diuretic, sedative, and antitussive activities [511], and the root
has been reported to have estrogenic activity [537–539]. Fukinolic acid [137] and formononetin [511]
have been reported to be estrogenic constituents of C. racemosa rhizome.
The traditional Cherokee
use of C. racemosa rhizome to stimulate menstruation [15] is consistent with the reported estrogenic
activity. There have been conflicting reports regarding the estrogenic activity of C. racemosa rhizome,
however [540–542], and a survey of 13 populations of C. racemosa in the eastern United States failed
to detect the presence of formononetin [543]. Molecular docking studies have suggested that C.
racemosa triterpenoids are unlikely estrogen receptor binding agents, but any estrogenic activity of C.
racemosa extract is probably due to phenolic components such as cimicifugic acid A, cimicifugic acid B,
cimicifugic acid G, cimiciphenol, cimiciphenone, cimiracemate A, cimiracemate B, cimiracemate C,
cimiracemate D, and fukinolic acid [544]. Although recent evidence suggests the estrogen receptor not
to be a target of C. racemosa phytochemical constituents, other biomolecular targets may be involved.
Rhizome extracts of C. racemosa have been shown to interact with the serotonin receptor [545], the
µ-opioid receptor [546,547] as well as the γ-aminobutryic acid type A (GABAA) receptors [548].
Modulation of these receptors may contribute to some of the biological effects of C. racemosa extracts.
Reviews of several randomized clinical trials have failed to demonstrate efficacy of C. racemosa on
menopausal symptoms [549,550]. However, one randomized, placebo-controlled double-blind clinical
trial with menopausal women, concluded that C. racemosa extract showed superiority over a placebo
in ameliorating menopausal disorders [551]. Clinical studies have generally suggested C. cimicifuga
use to be safe, but there have been some case reports indicating safety concerns [552].
The Cherokee have also used infusions of C. racemosa rhizome to treat rheumatism, coughs,
and colds [15]. Aqueous extracts of C. racemosa have demonstrated reduction of the release
of pro-inflammatory cytokines interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α), and
interferon-gamma (IFN-γ) in whole blood, and the prominent active component responsible was
isoferulic acid [553]. The ethyl acetate fraction of the aqueous extract of C. racemosa was also shown
to suppress the release of TNF-α, due to cimiracemate A [554]. Aqueous extracts reduced inducible
nitric oxide synthase (iNOS) protein expression as well as iNOS mRNA levels, but did not inhibit
iNOS enzymatic activity; the triterpenoid glycoside 23-epi-26-deoxyactein was found to be the active
principle in the extract [555]. These effects likely explain the anti-inflammatory activities of C. racemosaand their traditional uses to treat rheumatism and other inflammatory diseases.
Hamamelis virginiana L.
Hamamelis virginiana, American witch hazel, is a shrub or small tree, native to eastern North
America. Several Native American tribes have used the plant for numerous medicinal purposes.
Decoctions of the bark or the stems of witch hazel have been used as a topical lotion for cuts, bruises,
insect bites, external inflammations, and other skin problems [15]. In addition, the Cherokee people
took infusions of witch hazel for periodic pains, to treat colds, sore throats, and fevers. Modern
uses of witch hazel include treatment of hemorrhoids, inflammation of the mouth and pharynx
(leaf only), inflammation of the skin, varicose veins, wounds and burns [537]. Hamamelis virginiana
leaves contain up to 10% tannins, including gallic acid, polygalloylglucose, hamamelitannin and
analogs, flavonoids, and proanthocyanidins [511], which are responsible for the observed astringent,
anti-inflammatory, and hemostatic effects [537]. The bark also contains hamamelitannin and analogs,
and proanthocyanidins [511].
The aqueous ethanol extract of H. virginiana showed anti-inflammatory activity in the croton
oil mouse ear edema test [556] as well as the induced rat paw edema assay, confirming its use
as an anti-inflammatory agent [557]. The extract also showed notable antiviral activity against
Herpes simplex virus type 1 (HSV-1) [556]. Hamamelitannin and galloylated proanthocyanidins
from H. virginiana were found to be potent inhibitors of 5-lipoxygenase (5-LOX) [558]. Hamamelis
proanthocyanidins were found to stimulate cell growth of keratinocytes, enhancing cell growth, and
are likely responsible for the dermatological use of tannin-containing witch hazel preparations [559].
Hamamelis tannins have also shown cytotoxic activity against HT-29 human colorectal adenocarcinoma cells [223] and antiviral activity against influenza A virus and human papillomavirus [560].
The anti-inflammatory activity of witch hazel was demonstrated in a clinical study using a lotion
prepared from H. virginiana distillate, which showed suppression of erythema after ultraviolet (UVB)
light exposure [561]. Similarly, in a clinical trial with patients suffering from atopic eczema, a cream
containing H. virginiana distillate significantly reduced skin desquamation, itching and redness [562].
Of course, H. virginiana distillate will not contain tannins.
Hydrastis canadensis L.
Goldenseal (Hydrastis canadensis), a perennial herb in the Ranunculaceae, is native to eastern
North America from Ontario, Canada, south to Alabama and Georgia [563]. The Cherokee used the
root decoction of goldenseal as a tonic and wash for local inflammations; took the root decoction orally
to treat cancer, dyspepsia, and general debility [15]. Goldenseal is still used in herbal medicine to
control muscle spasms, treat cancer, increase blood pressure, treat gastrointestinal disorders, manage
painful and heavy menstruation, treat infections topically, and reduce swelling [537,564].
The major components in goldenseal root are isoquinoline alkaloids hydrastine, berberine, and
canadine, and berberine likely accounts for the biological activities of goldenseal. Berberine has
shown in vitro cytotoxic activity to HeLa human epitheloid cervix carcinoma, SK-OV-3 human
ovarian carcinoma, HEp2 human laryngeal carcinoma, HT-29 human colorectal adenocarcinoma,
MKN-45 human gastric cancer, HepG2 human hepatocellular carcinoma, MCF-7 and MDA-MB-231
human breast adenocarcinoma cell lines [565–568]. The cytotoxicity of berberine can be attributed
to DNA intercalation [569–571] and modulation of the human epidermal growth factor receptor 2
(HER2)/phosphatidylinositol-3-kinase (PI3K)/protein kinase B (Akt) signaling pathway [572,573].
Berberine has also shown antibacterial activity against Staphylococcus aureus [238,574], and Helicobacter pylori [453]; antiparasitic activity against Entamoeba histolytica, Giardia lamblia, Trichomonas vaginalis, Trypanosoma brucei, Trypanosoma congolense, Leishmania braziliensis panamensis, Leishmania major, and Plasmodium falciparum [575–578]; and anti-inflammatory activity in a serotonin-induced mouse paw edema assay [579]. In a randomized, double-blind, placebo-controlled clinical trial with patients suffering from acute watery diarrhea due to cholera, berberine showed a significant reduction in stool volume compared to the placebo [580]. Several clinical studies have demonstrated antihyperlipidemic effects of berberine in humans [581]
Juncus effusus L
Juncus effusus (common rush) is native to North and South America, Europe, Asia, and
Africa [563]. There are numerous varieties and subspecies of J. effusus with at least two in eastern
North America [582]. The Cherokee took a decoction of the plant as an emetic, while an infusion was
used to wash babies to strengthen them and prevent lameness [15]. In Chinese Traditional Medicine
(TCM), J. effusus is used as a sedative, anxiolytic, antipyretic, and to reduce swelling. Extracts of
J. effusus have revealed several cinnamoylglycerides [252,253], cycloartane triterpenoids [255–257],
phenanthrenes [258–264,266,267,269–272,583,584], and pyrenes [265,268]. Dehydroeffusol, effusol,
and juncusol, phenanthrenes isolated from J. effusus, have shown anxiolytic and sedative effects in a
mouse model [264,271], likely due to modulation of the gamma-amino butyric acid type A (GABAA)
receptor [272]. The GABAA modulatory activity may account for the TCM use of J. effusus as a sedative
and anxiolytic agent. Several J. effusus phenanthrenes have shown inhibition of NO production in
lipopolysaccharide (LPS)-activated murine macrophage RAW 264.7 cells, indicating anti-inflammatory
activity [270].
Panax quinquefolius L
American ginseng (Panax quinquefolius) is a member of the Araliaceae and is native to eastern
North America [585]. Ginseng root from P. ginseng or P. notoginseng, has been used for thousands
of years in the Asian traditional medicine. Panax quinquefolius is currently cultivated in the United
States, Canada, and China, and is used as a medical tonic worldwide. Native Americans have used P.
quinquefolius for numerous medical problems as well as a general tonic [15], and European settlers had
also utilized this plant for similar purposes [586]. The Cherokee used the root as an expectorant, to
treat colic, oral thrush, and as a general tonic [15].
The phytochemistry and pharmacology of P. quinquefolius has been reviewed several
times [333,339,341,342]. The major components in P. quinquefolius roots are triterpenoid glycosides,
the ginsenosides, as well as several polyacetylenes. The ginsenosides have shown anti-inflammatory,
antiproliferative, hepatoprotective, cardioprotective, neuroprotective, cholesterol-lowering, and
cognitive improvement [340].
Several clinical trials have been carried out using P. quinquefolius extracts. In terms of cognitive
function, a randomized, double-blind, placebo-controled crossover trial, P. quinquefolius extract showed significant improvement in working memory, choice reaction time and “calmness” [587]. A clinical trial to study the effects of P. quinquefolius extract on cancer-related fatigue showed a promising significant trend in relieving fatigue [588]. Panax quinquefolius extracts were found to be clinically effective in preventing upper respiratory infections in healthy adult senior citizens [589,590].
Sanguinaria canadensis L.
Bloodroot (Sanguinaria canadensis, Papaveraceae) is native to eastern North America [591]. The
plant has been used by Native Americans as a traditional medicine for a variety of ailments [455].
The Cherokee used a decoction of the root, in small doses, for coughs, lung inflammations, and
croup, and a root infusion was used as a wash for ulcers and sores [15]. The roots are rich in
isoquinoline alkaloids, including sanguinarine, chelerythrine, sanguilutine, chelilutine, sanguirubine,
chelirubine, protopine, and allocryptopine [455]. The traditional Cherokee uses of bloodroot as a cough
medicine/respiratory aid as well as for treating ulcers and sores can be attributed to the antimicrobial
activities of the isoquinoline alkaloids [592]. Thus, for example, sanguinarine has shown antimicrobial
activity against methicillin-resistant Staphylococcus aureus (MRSA) [593], biofilm-forming Candida
spp. [594], Mycobacterium spp. [452], and Helicobacter pylori [453].
Scutellaria lateriflora L
Infusions of the roots of blue skullcap (Scutellaria lateriflora, Lamiaceae) were used by the Cherokee
for monthly periods and to treat diarrhea; root decoctions were used as an emetic to expel afterbirth
and to remedy breast pains [15]. Interestingly, the aerial parts, rather than the roots, are currently used
as an herbal medicine as an anxiolytic, sedative and antispasmodic [511,537,595,596].
The phytochemistry and pharmacology of S. lateriflora have been reviewed [469].
The secondary metabolites from the aerial parts of S. lateriflora are dominated by flavonoid glycosides (baicalin, dihydrobaicalin, lateriflorin, ikonnikoside I, scutellarin (scutellarein-7-O-glucuronide), and oroxylin A-7-O-glucuronide, and 2-methoxy-chrysin-7-O-glucuronide), flavonoid aglycones (baicalein, oroxylin A, wogonin, and lateriflorein), phenylpropanoids (caffeic acid, cinnamic acid, p-coumaric acid, and ferulic acid), and clerodane diterpenoids (scutelaterin A, scutelaterin B, scutelaterin C, ajugapitin,
and scutecyprol A) [469]. The essential oil from the aerial parts of S. lateriflora (collected in northern
Iran) was composed largely of sesquiterpene hydrocarbons, δ-cadinene (27%), calamenene (15.2%),
β-elemene (9.2%), α-cubenene (4.2%), α-humulene (4.2%), and α-bergamotene (2.8%) [470].
The flavonoids scutellarin and baicalin and the phenylpropanoid ferulic acid have shown in vitro
estrogenic effects [597,598], and may be responsible for the traditional Cherokee uses of S. lateriflora.
Consistent with the current herbal medicinal use of S. lateriflora, the plant has shown
anti-convulsant activity in rodent models of acute seizures, attributable to the flavonoid
constituents [474]. Baicalin has shown anti-convulsant activity in pilocarpine-induced epileptic
model in rats [599], and wogonin has shown anti-convulsant effects on chemically-induced and
electroshock-induced seizures in rodents [600]. In addition, scutellarin has shown relaxant activity
using rodent aorta models [601,602], while wogonin showed smooth muscle relaxant activity in rat
aorta [603] and rat uterine smooth muscle [604]. On the other hand, both baicalin and baicalein
inhibited NO-mediated relaxation of rat aortic rings [605]. Baicalein and baicalin have shown
anxiolytic activity [606]. Apparently, baicalin and wogonin exert their anxiolytic effects through
allosteric modulation of the GABAA receptor by way of interaction at the benzodiazepine site [607,608].
Conversely, baicalein promotes anxiolytic effects via interaction with non-benzodiazepine sites of the
GABAA receptor [609]. There have apparently been no clinical trials on the root extracts of S. lateriflora.
However, in randomized, double-blind, placebo-controlled crossover clinical trials, the anxiolytic
effects of S. lateriflora herbal treatments significantly enhanced overall mood without reducing cognition
or energy [610,611]
Cherokee Aromatic Medicinal Plants and Their Phytochemical Constituents
Conclusions
This is not a complete list of the phytochemistry of Cherokee aromatic medicinal plants. Numerous
plants described in the Cherokee ethnobotanical literature [15–24] have not been investigated
for phytochemical constituents or pharmacological activity. In addition, in many instances the
phytochemistry is not sufficiently characterized, particularly in terms of the plant tissues used in
Cherokee traditional medicine. In this review, there are numerous instances where the phytochemical
constituents and the biological activities associated with them correlate with the traditional Cherokee
uses of the plant, but there are several instances where there is no apparent correlation. Therefore,
much work is needed to add to our knowledge of the pharmacological properties of the chemical
components, not to mention potential synergistic or antagonistic interactions.
Funding: This research received no external funding.
Acknowledgments: This work was carried out as part of the activities of the Aromatic Plant Research Center (APRC, https://aromaticplant.org/).
Conflicts of Interest: The author declares no conflict of interest.
References
1. Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The traditional medicine and modern medicine from natural products. Molecules 2016, 21, 559. [CrossRef] [PubMed]
2. Qin, G.; Xu, R. Recent advances on bioactive natural products from Chinese medicinal plants. Med. Res. Rev. 1998, 18, 375–382. [CrossRef]
3. Patwardhan, B.; Vaidya, A.D.B.; Chorghade, M. Ayurveda and natural products drug discovery. Curr. Sci. 2004, 86, 789–799.
4. Duke, J.A.; Bogenschutz-Godwin, M.J.; Ottesen, A.R. Duke’s Handbook of Medicinal Plants of Latin America; CRC Press: Boca Raton, FL, USA, 2009.
5. Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [CrossRef] [PubMed]
6. DeCorte, B.L. Underexplored opportunities for natural products in drug discovery. J. Med. Chem. 2016, 59, 9295–9304. [CrossRef] [PubMed]
7. Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [CrossRef] [PubMed]
8. Pinheiro, L.C.S.; Feitosa, L.M.; da Silveira, F.F.; Boechat, N. Current antimalarial therapies and advances
in the development of semi-synthetic artemisinin derivatives. An. Acad. Bras. Cienc. 2018, 90, 1251–1271. [CrossRef] [PubMed]
9. Bunkar, A.R. Therapeutic uses of Rauwolfia serpentina. Int. J. Adv. Sci. Res. 2017, 2, 23–26.
10. Monzote Fidalgo, L. Essential oil from Chenopodium ambrosioides as a promising antileishmanial agent. Nat.
Prod. Commun. 2007, 2, 1257–1262.
11. Cave, A.A. The Pequot War; University of Massachusetts Press: Amherst, MA, USA, 1996.
12. Roundtree, H.C. Pocahantas’s People: The Powhatan Indians of Virginia through Four Centuries; University of Oklahoma Press: Norman, OK, USA, 1990.
13. Ehle, J. Trail of Tears: The Rise and Fall of the Cherokee Nation; Anchor Books: New York, NY, USA, 1988.
14. Brown, D. Bury My Heart at Wounded Knee: An Indian History of the American West; Picador: New York, NY, USA, 2007.
15. Moerman, D.E. Native American Ethnobotany; Timber Press, Inc.: Portland, OR, USA, 1998.
16. Hutchens, A.R. Indian Herbalogy of North America; Shambala Publications: Boulder, CO, USA, 1991.
17. Hamel, P.B.; Chiltoskey, M.U. Cherokee Plants and Their Uses—A 400 Year History; Herald Publishing Company: Sylva, NC, USA, 1975.
18. Garrett, J.T. The Cherokee Herbal; Bear & Company: Rochester, VT, USA, 2003.
19. Mooney, J. The sacred formulas of the Cherokees. In Seventh Annual Report of the Bureau of Ethnology; Powell, J.W., Ed.; Government Printing Office: Washington, DC, USA, 1891; pp. 301–397.
20. Banks, W.H. Ethnobotany of the Cherokee Indians. Ph.D. Thesis, University of Tennessee, Knoxville, TN, USA, 1953.
21. Cozzo, D.N. Ethnobotanical Classifiction System and Medical Ethnobotany of the Eastern Band of the Cherokee Indians. Ph.D. Thesis, University of Georgia, Athens, GA, USA, 2004.
22. Winston, D. Nvwoti; Cherokee medicine and ethnobotany. J. Am. Herb. Guild 2001, 2, 45–49.
23. Core, E.L. Ethnobotany of the southern Appalachian Aborigines. Econ. Bot. 1967, 21, 199–214. [CrossRef]
24. Ray, L.E. Podophyllum peltatum and observations on the Creek and Cherokee Indians: William Bartram’s preservation of Native American pharmacology. Yale J. Biol. Med. 2009, 82, 25–36. [PubMed]
25. Vanhaelen, M.; Lejoly, J.; Hanocq, M.; Molle, L. Climatic and geographical aspects of medicinal plant
constituents. In The Medicinal Plant Industry; Wijesekera, R.O.B., Ed.; CRC Press: Boca Raton, FL, USA, 1991; pp. 59–76.
26. Royce, C.C. Map of the Former Territorial Limits of the Cherokee Nation of “Indians”; Map Showing the Territory Originally Assigned Cherokee “Nation of” Indians. Available online: https://www.loc.gov/item/99446145/ (accessed on 24 October 2018).
27. Abou-Zaid, M.M.; Nozzolillo, C. 1-O-galloyl-α-L-rhamnose from Acer rubrum. Phytochemistry 1999, 52, 1629–1631. [CrossRef]
28. Abou-Zaid, M.M.; Helson, B.V.; Nozzolillo, C.; Arnason, J.T. Ethyl m-digallate from red maple, Acer rubrum L., as the major resistance factor to forest tent caterpillar, Malacosoma disstria Hbn. J. Chem. Ecol. 2001, 27, 2517–2527. [CrossRef] [PubMed]
29. Ma, H. Phytochemical and Biological Investigation of Gallotannins from Red Maple (Acer rubrum) Species. Ph.D. Thesis, University of Rhode Island, Kingston, RI, USA, 2014.
30. Wan, C.; Yuan, T.; Xie, M.; Seeram, N.P. Acer rubrum phenolics include A-type procyanidins and a chalcone. Biochem. Syst. Ecol. 2012, 44, 1–3. [CrossRef]
31. Wan, C.; Yuan, T.; Li, L.; Kandhi, V.; Cech, N.B.; Xie, M.; Seeram, N.P. Maplexins, new α-glucosidase inhibitors from red maple (Acer rubrum) stems. Bioorg. Med. Chem. Lett. 2012, 22, 597–600. [CrossRef] [PubMed]
32. Yuan, T.; Wan, C.; Liu, K.; Seeram, N.P. New maplexins F-I and phenolic glycosides from red maple (Acer rubrum) bark. Tetrahedron 2012, 68, 959–964. [CrossRef]
33. González-Sarrías, A.; Yuan, T.; Seeram, N.P. Cytotoxicity and structure activity relationship studies of maplexins A–I, gallotannins from red maple (Acer rubrum). Food Chem. Toxicol. 2012, 50, 1369–1376.
[CrossRef] [PubMed]
34. Zhang, Y.; Ma, H.; Yuan, T.; Seeram, N.P. Red maple (Acer rubrum) aerial parts as a source of bioactive phenolics. Nat. Prod. Commun. 2015, 10, 1409–1412. [PubMed]
35. Bailey, A.E.; Asplund, R.O.; Ali, M.S. Isolation of methyl gallate as the antitumor principle of Acer saccharinum. J. Nat. Prod. 1986, 49, 1149–1150. [CrossRef] [PubMed]
36. Bin Muhsinah, A.; Ma, H.; DaSilva, N.A.; Yuan, T.; Seeram, N.P. Bioactive glucitol-core containing
gallotannins and other phytochemicals from silver maple (Acer saccharinum) leaves. Nat. Prod. Commun. 2017, 12, 83–84.
37. Falk, A.J.; Smolenski, S.J.; Bauer, L.; Bell, C.L. Isolation and identification of three new flavones from Achillea millefolium L. J. Pharm. Sci. 1975, 64, 1838–1842. [CrossRef] [PubMed]
38. Benetis, R.; Radušiene, J.; Janulis, V. Variability of phenolic compounds in flowers of ˙ Achillea millefolium wild populations in Lithuania. Medicina 2008, 44, 775–781. [CrossRef] [PubMed]
39. Glasl, S.; Mucaji, P.; Werner, I.; Presser, A.; Jurenitsch, J. Sesquiterpenes and flavonoid aglycones from a Hungarian taxon of the Achillea millefolium group. Z. Naturforsch. 2002, 57, 976–982. [CrossRef]
40. Vitalini, S.; Beretta, G.; Iriti, M.; Orsenigo, S.; Basilico, N.; Dall’Acqua, S.; Iorizzi, M.; Fico, G. Phenolic
compounds from Achillea millefolium L. and their bioactivity. Acta Biochim. Pol. 2011, 58, 203–209. [PubMed]
41. Dias, M.I.; Barros, L.; Dueñas, M.; Pereira, E.; Carvalho, A.M.; Alves, R.C.; Oliveira, M.B.P.P.;
Santos-Buelga, C.; Ferreira, I.C.F.R. Chemical composition of wild and commercial Achillea millefolium
L. and bioactivity of the methanolic extract, infusion and decoction. Food Chem. 2013, 141, 4152–4160.
[CrossRef] [PubMed]
42. Dall’Acqua, S.; Bolego, C.; Cignarella, A.; Gaion, R.M.; Innocenti, G. Vasoprotective activity of standardized
Achillea millefolium extract. Phytomedicine 2011, 18, 1031–1036. [CrossRef] [PubMed]
43. Tozyo, T.; Yoshimura, Y.; Sakurai, K.; Uchida, N.; Takeda, Y.; Nakai, H.; Ishi, H. Novel antitumor
sesquiterpenoids in Achillea millefolium. Chem. Pharm. Bull. 1994, 42, 1096–1100. [CrossRef] [PubMed]
44. Innocenti, G.; Vegeto, E.; Dall’Acqua, S.; Ciana, P.; Giorgetti, M.; Agradi, E.; Sozzi, A.; Fico, G.; Tomè, F.
In vitro estrogenic activity of Achillea millefolium L. Phytomedicine 2007, 14, 147–152. [CrossRef] [PubMed]
45. Pires, J.M.; Mendes, F.R.; Negri, G.; Duarte-Almeida, J.M.; Carlini, E.A. Antinociceptive peripheral effect of
Achillea millefolium L. and Artemisia vulgaris L.: Both plants known popularly by brand names of analgesic drugs. Phyther. Res. 2009, 23, 212–219. [CrossRef] [PubMed]
46. Guédon, D.; Abbe, P.; Lamaison, J.L. Leaf and flower head flavonoids of Achillea millefolium L. subspecies.
Biochem. Syst. Ecol. 1993, 21, 607–611. [CrossRef]
47. Csupor-Löffler, B.; Hajdú, Z.; Zupkó, I.; Réthy, B.; Falkay, G.; Forgo, P.; Hohmann, J. Antiproliferative
effect of flavonoids and sesquiterpenoids from Achillea millefolium s.l. on cultured human tumour cell lines. Phyther. Res. 2009, 23, 672–676. [CrossRef] [PubMed]
48. Candan, F.; Unlu, M.; Tepe, B.; Daferera, D.; Polissiou, M.; Sökmen, A.; Akpulat, H.A. Antioxidant and
antimicrobial activity of the essential oil and methanol extracts of Achillea millefolium subsp. millefolium Afan. (Asteraceae). J. Ethnopharmacol. 2003, 87, 215–220. [CrossRef]
49. Santoro, G.F.; Cardoso, M.G.; Gustavo, L.; Guimarães, L.G.L.; Mendonça, L.Z.; Soares, M.J. Trypanosoma cruzi:
Activity of essential oils from Achillea millefolium L., Syzygium aromaticum L. and Ocimum basilicum L. on epimastigotes and trypomastigotes. Exp. Parasitol. 2007, 116, 283–290. [CrossRef] [PubMed]
50. Bimbiraite, K.; Ragižinskien ˙ e, O.; Maruška, A.; Kornyšova, O. Comparison of the chemical composition of ˙ four yarrow (Achillea millefolium L.) morphotypes. Biologija 2008, 54, 208–212. [CrossRef]
51. Bocevska, M.; Sovová, H. Supercritical CO2 extraction of essential oil from yarrow. J. Supercrit. Fluids 2007, 40, 360–367. [CrossRef]
52. Barghamadi, A.; Mehrdad, M.; Sefidkon, F.; Yamini, Y.; Khajeh, M. Comparison of the volatiles of Achillea millefolium L. obtained by supercritical carbon dioxide extraction and hydrodistillation Methods. J. Essent. Oil Res. 2009, 21, 259–264. [CrossRef]
53. Figueiredo, A.C.; Barroso, J.G.; Pais, M.S.S.; Scheffer, J.J.C. Composition of the essential oils from leaves and flowers of Achillea millefolium L. ssp. millefolium. Flavour Fragr. J. 1992, 7, 219–222. [CrossRef]
54. Zhang, Z.; Li, S.; Zhang, S.; Gorenstein, D. Triterpenoid saponins from the fruits of Aesculus pavia.
Phytochemistry 2006, 67, 784–794. [CrossRef] [PubMed]
55. Zhang, Z.; Li, S. Cytotoxic triterpenoid saponins from the fruits of Aesculus pavia L. Phytochemistry 2007, 68, 2075–2086. [CrossRef] [PubMed]
56. Sun, Z.; Zhang, M.; Wu, Y.; Wan, A.; Zhang, R. Bioactive saponins from the fruits of Aesculus pavia L.
Fitoterapia 2011, 82, 1106–1109. [CrossRef] [PubMed]
57. Curir, P.; Galeotti, F.; Dolci, M.; Barile, E.; Lanzotti, V. Pavietin, a coumarin from Aesculus pavia with antifungal activity. J. Nat. Prod. 2007, 70, 1668–1671. [CrossRef] [PubMed]
58. Ferracini, C.; Curir, P.; Dolci, M.; Lanzotti, V.; Alma, A. Aesculus pavia foliar saponins: Defensive role against the leafminer Cameraria ohridella. Pest Manag. Sci. 2010, 66, 767–772. [CrossRef] [PubMed]
59. Lanzotti, V.; Termolino, P.; Dolci, M.; Curir, P. Paviosides A–H, eight new oleane type saponins from Aesculus pavia with cytotoxic activity. Bioorg. Med. Chem. 2012, 20, 3280–3286. [CrossRef] [PubMed]
60. Beier, R.C.; Norman, J.O.; Reagor, J.C.; Rees, M.S.; Mundy, B.P. Isolation of the major component in white snakeroot that is toxic after microsomal activation: Possible explanation of sporadic toxicity of white snakeroot plants and extracts. Nat. Toxins 1993, 1, 286–293. [CrossRef] [PubMed]
61. Lee, S.T.; Davis, T.Z.; Gerdner, D.R.; Stegelmeier, B.L.; Evans, T.J. Quantitative method for the measurement of three benzofuran ketones in rayless goldenrod (Isocoma pluriflora) and white snakeroot (Ageratina altissima) by high-performance liquid chromatography (HPLC). J. Agric. Food Chem. 2009, 57, 5639–5643. [CrossRef] [PubMed]
62. Lee, S.T.; Davis, T.Z.; Gardner, D.R.; Colegate, S.M.; Cook, D.; Green, B.T.; Meyerholtz, K.A.; Wilson, C.R.; Stegelmeier, B.L.; Evans, T.J. Tremetone and structurally related compounds in white snakeroot (Ageratina altissima): A plant associated with trembles and milk sickness. J. Agric. Food Chem. 2010, 58, 8560–8565. [CrossRef] [PubMed]
63. Fritsch, R.M.; Keusgen, M. Occurrence and taxonomic significance of cysteine sulphoxides in the genus Allium L. (Alliaceae). Phytochemistry 2006, 67, 1127–1135. [CrossRef] [PubMed]
64. Sobolewska, D.; Michalska, K.; Podolak, I.; Grabowska, K. Steroidal saponins from the genus Allium.
Phytochem. Rev. 2016, 15, 1–35. [CrossRef] [PubMed]
65. Calvey, E.M.; White, K.D.; Matusik, J.E.; Sha, D.; Block, E. Allium chemistry: Identification of organosulfur compounds in ramp (Allium tricoccum) homogenates. Phytochemistry 1998, 49, 359–364. [CrossRef]
66. Chen, S.; Snyder, J.K. Molluscicidal saponins from Allium vineale. Tetrahedron Lett. 1987, 28, 5603–5606. [CrossRef]
67. Chen, S.; Snyder, J.K. Diosgenin-bearing mulluscicidal saponins from Allium vineale: An NMR approach for the structural assignment of oligosaccharide units. J. Org. Chem. 1989, 54, 3679–3689. [CrossRef]
68. Demirtas, I.; Erenler, R.; Elmastas, M.; Goktasoglu, A. Studies on the antioxidant potential of flavones of Allium vineale isolated from its water-soluble fraction. Food Chem. 2013, 136, 34–40. [CrossRef] [PubMed]
69. Satyal, P.; Craft, J.D.; Dosoky, N.S.; Setzer, W.N. The chemical compositions of the volatile oils of garlic (Allium sativum) and wild garlic (Allium vineale). Foods 2017, 6, 63. [CrossRef] [PubMed]
70. Li, H.; O’Neill, T.; Webster, D.; Johnson, J.A.; Gray, C.A. Anti-mycobacterial diynes from the Canadian medicinal plant Aralia nudicaulis. J. Ethnopharmacol. 2012, 140, 141–144. [CrossRef] [PubMed]
71. Davé, P.C.; Vogler, B.; Setzer, W.N. Chemical compositions of the leaf essential oils of Aralia spinosa from three habitats in Northern Alabama. Am. J. Plant Sci. 2011, 02, 507–510. [CrossRef]
72. Wolf, S.J.; Denford, K.E. Flavonoid variation in Arnica cordifolia: An apomictic polyploid complex. Biochem. Syst. Ecol. 1983, 11, 111–114. [CrossRef]
73. Merfort, I.; Wendisch, D. Sesquiterpene lactones of Arnica cordifolia, subgenus austromontana. Phytochemistry 1993, 34, 1436–1437. [CrossRef]
74. Nematollahi, F.; Rustaiyan, A.; Larijani, K.; Madimi, M.; Masoudi, S. Essential oil composition of Artemisia biennis Willd. and Pulicaria undulata (L.) C.A. Mey., two Compositae herbs growing wild in Iran. J. Essent. Oil Res. 2006, 18, 339–341. [CrossRef]
75. Lopes-Lutz, D.; Alviano, D.S.; Alviano, C.S.; Kolodziejczyk, P.P. Screening of chemical composition,
antimicrobial and antioxidant activities of Artemisia essential oils. Phytochemistry 2008, 69, 1732–1738. [CrossRef] [PubMed]
76. Jeong, S.Y.; Jun, D.Y.; Kim, Y.H.; Min, B.-S.; Min, B.K.; Woo, M.H. Monoterpenoids from the aerial parts of Aruncus dioicus var. kamtschaticus and their antioxidant and cytotoxic activities. Bioorg. Med. Chem. Lett. 2011, 21, 3252–3256. [PubMed]
77. Han, C.R.; Jun, D.Y.; Woo, H.J.; Jeong, S.-Y.; Woo, M.-H.; Kim, Y.H. Induction of microtubule-damage, mitotic arrest, Bcl-2 phosphorylation, Bak activation, and mitochondria-dependent caspase cascade is involved in human Jurkat T-cell apoptosis by aruncin B from Aruncus dioicus var. kamtschaticus. Bioorg. Med. Chem. Lett. 2012, 22, 945–953. [CrossRef] [PubMed]
78. Zhao, B.T.; Jeong, S.Y.; Vu, V.D.; Min, B.S.; Kim, Y.H.; Woo, M.H. Cytotoxic and anti-oxidant constituents from the aerial parts of Aruncus dioicus var. kamtschaticus. Nat. Prod. Sci. 2013, 19, 6670.
79. Vo, Q.H.; Nguyen, P.H.; Zhao, B.T.; Thi, Y.N.; Nguyen, D.H.; Kim, W.I.; Seo, U.M.; Min, B.S.; Woo, M.H.
Bioactive constituents from the n-butanolic fraction of Aruncus dioicus var. kamtschaticus. Nat. Prod. Sci. 2014,20, 274–280
80. Fusani, P.; Piwowarski, J.P.; Zidorn, C.; Kiss, A.K.; Scartezzini, F.; Granica, S. Seasonal variation in secondary metabolites of edible shoots of Buck’s beard [Aruncus dioicus (Walter) Fernald (Rosaceae)]. Food Chem. 2016, 202, 23–30. [CrossRef] [PubMed]
81. Iwashina, T.; Kitajima, J. Chalcone and flavonol glycosides from Asarum canadense (Aristolochiaceae). Phytochemistry 2000, 55, 971–974. [CrossRef]
82. Bauer, L.; Bell, C.L.; Gearien, J.E.; Takeda, H. Constituents of the rhizome of Asarum canadense. J. Pharm. Sci. 1967, 56, 336–343. [CrossRef]
83. Motto, M.G.; Secord, N.J. Composition of the essential oil from Asarum canadense. J. Agric. Food Chem. 1985, 33, 789–791. [CrossRef]
84. Bélanger, A.; Collin, G.; Garneau, F.-X.; Gagnon, H.; Pichette, A. Aromas from Quebec. II. Composition of the essential oil of the rhizomes and roots of Asarum canadense L. J. Essent. Oil Res. 2010, 22, 164–169. [CrossRef]
85. Garneau, F.; Collin, G.; Gagnon, H. Chemical composition and stability of the hydrosols obtained during essential oil production. I. The case of Melissa officinalis L. and Asarum canadense L. Am. J. Essent. Oils Nat. Prod. 2014, 2, 54–62.
86. Abe, F.; Yamauchi, T. An androstane bioside and 3’-thiazolidinone derivatives of doubly-linked cardenolide glycosides from the roots of Asclepias tuberosa. Chem. Pharm. Bull. 2000, 48, 991–993. [CrossRef] [PubMed]
87. Abe, F.; Yamauchi, T. Pregnane glycosides from the roots of Asclepias tuberosa. Chem. Pharm. Bull. 2000, 48, 1017–1022. [CrossRef] [PubMed]
88. Warashina, T.; Noro, T. 8,14-Secopregnane glycosides from the aerial parts of Asclepias tuberosa. Phytochemistry 2009, 70, 1294–1304. [CrossRef] [PubMed]
89. Warashina, T.; Noro, T. 8,12;8,20-Diepoxy-8,14-secopregnane glycosides from the aerial parts of Asclepias tuberosa. Chem. Pharm. Bull. 2010, 58, 172–179. [CrossRef] [PubMed]
90. Warashina, T.; Umehara, K.; Miyase, T.; Noro, T. 8,12;8,20-Diepoxy-8,14-secopregnane glycosides from roots of Asclepias tuberosa and their effect on proliferation of human skin fibroblasts. Phytochemistry 2011, 72, 1865–1875. [CrossRef] [PubMed]
91. Lebreton, P.; Markham, K.R.; Swift, W.T., III; Mabry, T.J. Flavonoids of Baptista australis (Leguminosae). Phytochemistry 1967, 6, 1675–1680. [CrossRef]
92. Markham, K.R.; Swift, W.T.; Mabry, T.J. A new isoflavone glycoside from Baptisia australis. J. Org. Chem. 1968, 33, 462–464. [CrossRef] [PubMed]
93. Fraser, A.M.; Robins, D.J. Incorporation of enantiomeric [1 2H]cadaverines into the quinolizindine alkaloids (+)-sparteine and (-)-N-methylcytisine in Baptisia australis. J. Chem. Soc. Chem. Commun. 1986, 1986, 545–547. [CrossRef]
94. Zenk, M.H.; Rueffer, M.; Amann, M.; Deus-Neumann, B. Benzylisoquinoline biosynthesis by cultivated plant cells and isolated enzymes. J. Nat. Prod. 1985, 48, 725–738. [CrossRef]
95. Woods, K.E.; Jones, C.D.; Setzer, W.N. Bioactivities and compositions of Betula nigra essential oils. J. Med. Act. Plants 2013, 2, 1–9.
96. Hua, Y.; Bentley, M.D.; Cole, B.J.W.; Murray, K.D.; Alford, A.R. Triterpenes from the outer bark of Betula nigra. J. Wood Chem. Technol. 1991, 11, 503–516. [CrossRef]
97. Wollenweber, E. Rare methoxy flavonoids from buds of Betula nigra. Phytochemistry 1976, 15, 438–439.[CrossRef]
98. Wollenweber, E. New flavonoids from Betula nigra. Phytochemistry 1977, 16, 295. [CrossRef]
99. Tellez, M.R.; Dayan, F.E.; Schrader, K.K.; Wedge, D.E.; Duke, S.O. Composition and some biological activitiesof the essential oil of Callicarpa americana (L.). J. Agric. Food Chem. 2000, 48, 3008–3012. [CrossRef] [PubMed]
100. Cantrell, C.L.; Klun, J.A.; Bryson, C.T.; Kobaisy, M.; Duke, S.O. Isolation and identification of mosquito bite deterrent terpenoids from leaves of American (Callicarpa americana) and Japanese (Callicarpa japonica) beautyberry. J. Agric. Food Chem. 2005, 53, 5948–5953. [CrossRef] [PubMed]
101. Carroll, J.F.; Cantrell, C.L.; Klun, J.A.; Kramer, M. Repellency of two terpenoid compounds isolated from Callicarpa americana (Lamiaceae) against Ixodes scapularis and Amblyomma americanum ticks. Exp. Appl. Acarol. 2007, 41, 215–224. [CrossRef] [PubMed]
102. Jones, W.P.; Lobo-Echeverri, T.; Mi, Q.; Chai, H.-B.; Soejarto, D.D.; Cordell, G.A.; Swanson, S.M.;
Kinghorn, A.D. Cytotoxic constituents from the fruiting branches of Callicarpa americana collected in southern Florida. J. Nat. Prod. 2007, 70, 372–377. [CrossRef] [Pub
103. Collins, R.P.; Chang, N.; Knaak, L.E. Anthocyanins in Calycanthus floridus. Am. Midl. Nat. 1969, 82, 633–637.
[CrossRef]
104. Miller, E.R.; Taylor, G.W.; Eskew, M.H. The volatile oil of Calycanthus floridus. J. Am. Chem. Soc. 1914, 36,
2182–2187. [CrossRef]
105. Collins, R.P.; Halim, A.F. Essential leaf oils in Calycanthus floridus. Planta Med. 1971, 20, 241–243. [CrossRef]
[PubMed]
106. Akhlaghi, H. Chemical composition of the essential oil from flowers of Calycanthus floridus L. var. oblongifolius
(Nutt.) D.E. Boufford & S.A. Spongberg from Iran. J. Pharm. Heal. Sci. 2014, 2, 111–114.
107. Akhlaghi, H. Chemical composition of the essential oil from stems of Calycanthus floridus L. var. oblongifolius
from Iran. Chem. Nat. Compd. 2008, 44, 661–662. [CrossRef]
108. Woldemariam, T.Z.; Betz, J.M.; Houghton, P.J. Analysis of aporphine and quinolizidine alkaloids from
Caulophyllum thalictroides by densitometry and HPLC. J. Pharm. Biomed. Anal. 1997, 15, 839–843. [CrossRef]
109. Betz, J.M.; Andrzejewski, D.; Troy, A.; Casey, R.E.; Obermeyer, W.R.; Page, S.W.; Woldemariam, T.Z. Gas
chromatographic determination of toxic quinolizidine alkaloids in blue cohosh Caulophyllum thalictroides (L.)
Michx. Phytochem. Anal. 1998, 9, 232–236. [CrossRef]
110. Kennelly, E.J.; Flynn, T.J.; Mazzola, E.P.; Roach, J.A.; McCloud, T.G.; Danford, D.E.; Betz, J.M. Detecting
potential teratogenic alkaloids from blue cohosh rhizomes using an in vitro rat embryo culture. J. Nat. Prod.
1999, 62, 1385–1389. [CrossRef] [PubMed]
111. Ali, Z.; Khan, I.A. Alkaloids and saponins from blue cohosh. Phytochemistry 2008, 69, 1037–1042. [CrossRef]
[PubMed]
112. Madgula, V.L.M.; Ali, Z.; Smillie, T.; Khan, I.; Walker, L.A.; Khan, S.I. Alkaloids and saponins as cytochrome
P450 inhibitors from blue cohosh (Caulophyllum thalictroides) in an in vitro assay. Planta Med. 2009, 75,
329–332. [CrossRef] [PubMed]
113. Jhoo, J.-W.; Sang, S.; He, K.; Cheng, X.; Zhu, N.; Stark, R.E.; Zheng, Q.Y.; Rosen, R.T.; Ho, C.-T.
Characterization of the triterpene saponins of the roots and rhizomes of blue cohosh (Caulophyllum
thalictroides). J. Agric. Food Chem. 2001, 49, 5969–5974. [CrossRef] [PubMed]
114. Matsuo, Y.; Watanabe, K.; Mimaki, Y. Triterpene glycosides from the underground parts of Caulophyllum
thalictroides. J. Nat. Prod. 2009, 72, 1155–1160. [CrossRef] [PubMed]
115. Lee, Y.; Jung, J.-C.; Ali, Z.; Khan, I.A.; Oh, S. Anti-inflammatory effect of triterpene saponins isolated from
blue cohosh (Caulophyllum thalictroides). Evid. Based Complement. Altern. Med. 2012, 2012, 798192. [CrossRef]
[PubMed]
116. Warnhoff, E.W.; Pradhan, S.K.; Ma, J.C. Ceanothus alkaloids I. Isolation, separation, and characterization. Can.
J. Chem. 1965, 53, 2594–2602. [CrossRef]
117. Klein, F.K.; Rapoport, H. Ceanothus alkaloids. Americine. J. Am. Chem. Soc. 1968, 90, 2398–2404. [CrossRef]
[PubMed]
118. Servis, R.E.; Kosak, A.I.; Tschesche, R.; Frohberg, E.; Fehlhaber, H.-W. Peptide alkaloids from Ceanothus
americanus L. (Rhamnaceae). J. Am. Chem. Soc. 1969, 91, 5619–5624. [CrossRef]
119. Steinberg, K.M.; Satyal, P.; Setzer, W.N. Chemical composition of the bark essential oil of Cercis canadensis L.
(Fabaceae). Am. J. Essent. Oils Nat. Prod. 2017, 5, 15–17.
120. Bowers, M.D.; Boockvar, K.; Collinge, S.K. Iridoid glycosides of Chelone glabra (Scrophulariaceae) and their
sequestration by larvae of a wawfly, Tenthredo grandis (Tenthredinidae). J. Chem. Ecol. 1993, 19, 815–823.
[CrossRef] [PubMed]
121. St. Pyrek, J. Sesquiterpene lactones of Cinchorium intybus and Leontodon autumnalis. Phytochemistry 1985, 24,
186–188. [CrossRef]
122. Kisiel, W.; Zieli´nska, K. Guaianolides from Cichorium intybus and structure revision of Cichorium
sesquiterpene lactones. Phytochemistry 2001, 57, 523–527. [CrossRef]
123. Bischoff, T.A.; Kelley, C.J.; Karchesy, Y.; Laurantos, M.; Nguyen-Dinh, P.; Arefi, A.G. Antimalarial activity of
lactucin lnd lactucopicrin: Sesquiterpene lactones isolated from Cichorium intybus L. J. Ethnopharmacol. 2004,
95, 455–457. [CrossRef] [PubMed]
124. Wesołowska, A.; Nikiforuk, A.; Michalska, K.; Kisiel, W.; Chojnacka-Wójcik, E. Analgesic and sedative
activities of lactucin and some lactucin-like guaianolides in mice. J. Ethnopharmacol. 2006, 107, 254–258.
[CrossRef] [PubMed]
125. Nørbæk, R.; Nielsen, K.; Kondo, T. Anthocyanins from flowers of Cichorium intybus. Phytochemistry 2002, 60,
357–359. [CrossRef]
126. He, K.; Zheng, B.; Kim, C.H.; Rogers, L.; Zheng, Q. Direct analysis and identification of triterpene glycosides
by LC/MS in black cohosh, Cimicifuga racemosa, and in several commercially available black cohosh products.
Planta Med. 2000, 66, 635–640. [CrossRef] [PubMed]
127. Bedir, E.; Khan, I.A. Cimiracemoside A: A new cyclolanostanol xyloside from the rhizome of Cimicifuga
racemosa. Chem. Pharm. Bull. 2000, 48, 425–427. [CrossRef] [PubMed]
128. Lai, G.F.; Wang, Y.-F.; Fan, L.-M.; Cao, J.-X.; Luo, S.-D. Triterpenoid glycoside from Cimicifuga racemosa. J.
Asian Nat. Prod. Res. 2005, 7, 695–699. [CrossRef] [PubMed]
129. Shao, Y.; Harris, A.; Wang, M.; Zhang, H.; Cordell, G.A.; Bowman, M.; Lemmo, E. Triterpene glycosides from
Cimicifuga racemosa. J. Nat. Prod. 2000, 63, 905–910. [CrossRef] [PubMed]
130. Watanabe, K.; Mimaki, Y.; Sakagami, H.; Sashida, Y. Cycloartane glycosides from the rhizomes of Cimicifuga
racemosa and their cytotoxic activities. Chem. Pharm. Bull. 2002, 50, 121–125. [CrossRef] [PubMed]
131. Tsukamoto, S.; Aburatani, M.; Ohta, T. Isolation of CYP3A4 inhibitors from the black cohosh (Cimicifuga
racemosa). Evid. Based Complement. Altern. Med. 2005, 2, 223–226. [CrossRef] [PubMed]
132. Cicek, S.S.; Schwaiger, S.; Ellmerer, E.P.; Stuppner, H. Development of a fast and convenient method for the
isolation of triterpene saponins from Actaea racemosa by high-speed countercurrent chromatography coupled
with evaporative light scattering detection. Planta Med. 2010, 76, 467–473. [CrossRef] [PubMed]
133. Jamróz, M.K.; Jamróz, M.H.; Dobrowolski, J.C.; Gli´nski, J.A.; Davey, M.H.; Wawer, I. Novel and unusual
triterpene from black cohosh. Determination of structure of 9,10-seco-9,19-cyclolanostane xyloside
(cimipodocarpaside) by NMR, IR and Raman spectroscopy and DFT calculations. Spectrochim. Acta Part A
Mol. Biomol. Spectrosc. 2011, 78, 107–112. [CrossRef] [PubMed]
134. Jamróz, M.K.; Paradowska, K.; Gli´nski, J.A.; Wawer, I. 13C CPMAS NMR studies and DFT calculations of
triterpene xylosides isolated from Actaea racemosa. J. Mol. Struct. 2011, 994, 248–255. [CrossRef]
135. Jamróz, M.K.; Jamróz, M.H.; Dobrowolski, J.C.; Gli´nski, J.A.; Gle´nsk, M. One new and six known triterpene
xylosides from Cimicifuga racemosa: FT-IR, Raman and NMR studies and DFT calculations. Spectrochim. Acta
Part A Mol. Biomol. Spectrosc. 2012, 93, 10–18. [CrossRef] [PubMed]
136. He, C.-C.; Dai, Y.-Q.; Hui, R.-R.; Hua, J.; Chen, H.-J.; Luo, Q.-Y.; Li, J.-X. NMR-based metabonomic approach
on the toxicological effects of a Cimicifuga triterpenoid. J. Appl. Toxicol. 2012, 32, 88–97. [CrossRef] [PubMed]
137. Kruse, S.O.; Löhning, A.; Pauli, G.F.; Winterhoff, H.; Nahrstedt, A. Fukiic and piscidic acid esters from the
rhizome of Cimicifuga racemosa and the in vitro estrogenic activity of fukinolic acid. Planta Med. 1999, 65,
763–764. [CrossRef] [PubMed]
138. Stromeier, S.; Petereit, F.; Nahrstedt, A. Phenolic esters from the rhizomes of Cimicifuga racemosa do not cause
proliferation effects in MCF-7 cells. Planta Med. 2005, 71, 495–500. [CrossRef] [PubMed]
139. Chen, S.-N.; Fabricant, D.S.; Lu, Z.-Z.; Zhang, H.; Fong, H.H.S.; Farnsworth, N.R. Cimiracemates A-D,
phenylpropanoid esters from the rhizomes of Cimicifuga racemosa. Phytochemistry 2002, 61, 409–413.
[CrossRef]
140. Li, W.; Chen, S.; Fabricant, D.; Angerhofer, C.K.; Fong, H.S.; Farnsworth, N.R.; Fitzloff, J.F. High-performance
liquid chromatographic analysis of black cohosh (Cimicifuga racemosa) constituents with in-line evaporative
light scattering and photodiode array detection. Anal. Chim. Acta 2002, 471, 61–75. [CrossRef]
141. Nuntanakorn, P.; Jiang, B.; Einbond, L.S.; Yang, H.; Kronenberg, F.; Weinstein, I.B.; Kennelly, E.J. Polyphenolic
constituents of Actaea racemosa. Nournal Nat. Prod. 2006, 69, 314–318. [CrossRef] [PubMed]
142. Gödecke, T.; Lankin, D.C.; Nikolic, D.; Chen, S.-N.; van Breemen, R.B.; Farnsworth, N.R.; Pauli, G.F.
Guanidine alkaloids and Pictet-Spengler adducts from black cohosh (Cimicifuga racemosa). J. Nat. Prod. 2009,
72, 433–437. [CrossRef] [PubMed]
143. Azimova, S.S.; Gluchenkova, A.I. (Eds.) Collinsonia canadensis L. In Lipids, Lipophilic Components and Essential
Oils from Plant Sources; Springer: London, UK, 2012; p. 401.
144. Joshi, B.S.; Moore, K.M.; Pelletier, S.W.; Puar, M.S.; Pramanik, B.N. Saponins from Collinsonia canadensis. J.
Nat. Prod. 1992, 55, 1468–1474. [CrossRef]
145. Stevens, J.F.; Ivancic, M.; Deinzer, M.L.; Wollenweber, E. A novel 2-hydroxyflavanone from Collinsonia
canadensis. J. Nat. Prod. 1999, 62, 392–394. [CrossRef] [PubMed]
146. Hutton, K. A Comparative Study of the Plants Used for Medicinal Purposes by the Creek and Seminole
Tribes. Master’s Thesis, University of South Florida, Tampa, FL, USA, 2010.
147. Mukhtar, N.; Iqbal, K.; Anis, I.; Malik, A. Sphingolipids from Conyza canadensis. Phytochemistry 2002, 61,
1005–1008. [CrossRef]
148. Mukhtar, N.; Iqbal, K.; Malik, A. Sphingolipids from Conyza canadensis. Chem. Pharm. Bull. 2002, 50,
1558–1560. [CrossRef] [PubMed]
149. Yan, M.M.; Li, T.Y.; Zhao, D.Q.; Shao, S.; Bi, S.N. A new derivative of triterpene with anti-melanoma B16
activity from Conyza canadensis. Chin. Chem. Lett. 2010, 21, 834–837. [CrossRef]
150. Shakirullah, M.; Ahmad, H.; Shah, M.R.; Imtiaz, A.; Ishaq, M.; Khan, N.; Badshah, A.; Khan, I. Antimicrobial
activities of conyzolide and conyzoflavone from Conyza canadensis. J. Enzyme Inhib. Med. Chem. 2011, 26,
468–471. [CrossRef] [PubMed]
151. Xie, W.D.; Gao, X.; Jia, Z.J. A new C-10 acetylene and a new triterpenoid from Conyza canadensis. Arch. Pharm.
Res. 2007, 30, 547–551. [CrossRef] [PubMed]
152. Ding, Y.; Su, Y.; Guo, H.; Yang, F.; Mao, H.; Gao, X.; Zhu, Z.; Tu, G. Phenylpropanoyl esters from horseweed
(Conyza canadensis) and their inhibitory effects on catecholamine secretion. J. Nat. Prod. 2010, 73, 270–274.
[CrossRef] [PubMed]
153. Queiroz, S.C.N.; Cantrell, C.L.; Duke, S.O.; Nandula, V.; Moraes, R.M.; Cerdeira, A.L. Bioassay-directed
isolation and identification of phytotoxic terpenoids from horseweed (Conyza canadensis). Planta Med. 2012,
78, P48. [CrossRef]
154. Porto, R.S.; Rath, S.; Queiroz, S.C.N. Conyza canadensis: Green extraction method of bioactive compounds
and evaluation of their antifungal activity. J. Braz. Chem. Soc. 2017, 28, 913–919. [CrossRef]
155. Pawlaczyk, I.; Czerchawski, L.; Kuliczkowski, W.; Karolko, B.; Pilecki, W.; Witkiewicz, W.; Gancarz, R.
Anticoagulant and anti-platelet activity of polyphenolic-polysaccharide preparation isolated from the
medicinal plant Erigeron canadensis L. Thromb. Res. 2011, 127, 328–340. [CrossRef] [PubMed]
156. Csupor-Löffler, B.; Hajdú, Z.; Zupkó, I.; Molnár, J.; Forgo, P.; Kele, Z.; Hohmann, J. New dihydropyrone
derivatives and further antitumor compounds from Conyza canadensis. Planta Med. 2010, 76, P258. [CrossRef]
157. Csupor-Löffler, B.; Hajdú, Z.; Zupkó, I.; Molnár, J.; Forgo, P.; Vasas, A.; Kele, Z.; Hohmann, J. Antiproliferative
constituents of the roots of Conyza canadensis. Planta Med. 2011, 77, 1183–1188. [CrossRef] [PubMed]
158. Liu, K.; Qin, Y.-H.; Yu, J.-Y.; Ma, H.; Song, X.-L. 3-β-Erythrodiol isolated from Conyza canadensis inhibits
MKN-45 human gastric cancer cell proliferation by inducing apoptosis, cell cycle arrest, DNA fragmentation,
ROS generation and reduces tumor weight and volume in mouse xenograft model. Oncol. Rep. 2016, 35,
2328–2338. [CrossRef] [PubMed]
159. Banday, J.A.; Mir, F.A.; Farooq, S.; Qurishi, M.A.; Koul, S.; Razdan, T.K. Salicylic acid and methyl gallate
from the roots of Conyza canedensis. Int. J. Chem. Anal. Sci. 2012, 3, 2–5.
160. Banday, J.A.; Farooq, S.; Qurishi, M.A.; Koul, S.; Razdan, T.K. Conyzagenin-A and B, two new epimeric
lanostane triterpenoids from Conyza canadensis. Nat. Prod. Res. 2013, 27, 975–981. [CrossRef] [PubMed]
161. Curini, M.; Bianchi, A.; Epifano, F.; Bruni, R.; Torta, L.; Zambonelli, A. Compsotion and in vitro antifungal
activity of essential oils of Erigeron canadensis and Myrtus communis from France. Chem. Nat. Compd. 2003, 39,
191–194. [CrossRef]
162. Lis, A.; Piggott, J.R.; Góra, J. Chemical composition variability of the essential oil of Conyza canadensis Cronq.
Flavour Fragr. J. 2003, 18, 364–367. [CrossRef]
163. Tzakou, O.; Vagias, C.; Gani, A.; Yannitsaros, A. Volatile constituents of essential oils isolated at different
growth stages from three Conyza species growing in Greece. Flavour Fragr. J. 2005, 20, 425–428. [CrossRef]
164. Lis, A.; Góra, J. Essential oil of Conyza canadensis (L.) Cronq. J. Essent. Oil Res. 2000, 12, 781–783. [CrossRef]
165. Stoyanova, A.; Georgiev, E.; Kermedchieva, D.; Lis, A.; Gora, J. Changes in the essential oil of Conyza
canadensis (L.) Cronquist. during its vegetation. J. Essent. Oil Res. 2003, 15, 44–45. [CrossRef]
166. Rustaiyan, A.; Azar, P.A.; Moradalizadeh, M.; Masoudi, S.; Ameri, N. Volatile constituents of three
Compositae herbs: Anthemis altissima L. var altissima, Conyza canadensis (L.) Cronq. and Grantina aucheri
Boiss. growing wild in Iran. J. Essent. Oil Res. 2004, 16, 579–581. [CrossRef]
167. Miyazawa, M.; Yamamoto, K.; Kameoka, H. The essential oil of Erigeron canadensis L. J. Essent. Oil Res. 1992,
4, 227–230. [CrossRef]
168. Choi, H.-J.; Want, H.-Y.; Kim, Y.-N.; Heo, S.-J.; Kim, N.-K.; Jeong, M.-S.; Park, Y.-H.; Kim, S. Composition and
cytotoxicity of essential oil extracted by steam distillation from horseweed (Erigeron canadensis L.) in Korea. J.
Korean Soc. Appl. Biol. Chem. 2008, 51, 55–59.
169. Veres, K.; Csupor-Löffler, B.; Lázár, A.; Hohmann, J. Antifungal activity and composition of essential oils of
Conyza canadensis herbs and roots. Sci. World J. 2012, 2012. [CrossRef] [PubMed]
170. Liu, Y.; Du, D.; Liang, Y.; Xin, G.; Huang, B.-Z.; Huang, W. Novel polyacetylenes from Coreopsis tinctoria Nutt.
J. Asian Nat. Prod. Res. 2015, 17, 744–749. [CrossRef] [PubMed]
171. Lam, S.-C.; Lam, S.-F.; Zhao, J.; Li, S.-P. Rapid identification and comparison of compounds with antioxidant
activity in Coreopsis tinctoria herbal tea by high-performance thin-layer chromatography coupled with DPPH
bioautography and densitometry. J. Food Sci. 2016, 81, C2218–C2223. [CrossRef] [PubMed]
172. Zhang, Y.; Shi, S.; Zhao, M.; Chai, X.; Tu, P. Coreosides A-D, C14-polyacetylene glycosides from the capitula
of Coreopsis tinctoria and its anti-inflammatory activity against COX-2. Fitoterapia 2013, 87, 93–97. [CrossRef]
[PubMed]
173. Guo, J.; Wang, A.; Yang, K.; Ding, H.; Hu, Y.; Yang, Y.; Huang, S.; Xu, J.; Liu, T.; Yang, H.; et al. Isolation,
characterization and antimicrobial activities of polyacetylene glycosides from Coreopsis tinctoria Nutt.
Phytochemistry 2017, 136, 65–69. [CrossRef] [PubMed]
174. Du, D.; Jin, T.; Xing, Z.-H.; Hu, L.-Q.; Long, D.; Li, S.-F.; Gong, M. One new linear C14 polyacetylene glucoside
with antiadipogenic activities on 3T3-L1 cells from the capitula of Coreopsis tinctoria. J. Asian Nat. Prod. Res.
2016, 18, 784–790. [CrossRef] [PubMed]
175. Dias, T.; Liu, B.; Jones, P.; Houghton, P.J.; Mota-Filipe, H.; Paulo, A. Cytoprotective effect of Coreopsis tinctoria
extracts and flavonoids on tBHP and cytokine-induced cell injury in pancreatic MIN6 cells. J. Ethnopharmacol.
2012, 139, 485–492. [CrossRef] [PubMed]
176. Zhang, Y.; Shi, S.; Zhao, M.; Jiang, Y.; Tu, P. A novel chalcone from Coreopsis tinctoria Nutt. Biochem. Syst. Ecol.
2006, 34, 766–769. [CrossRef]
177. Dias, T.; Bronze, M.R.; Houghton, P.J.; Mota-Filipe, H.; Paulo, A. The flavonoid-rich fraction of Coreopsis
tinctoria promotes glucose tolerance regain through pancreatic function recovery in streptozotocin-induced
glucose-intolerant rats. J. Ethnopharmacol. 2010, 132, 483–490. [CrossRef] [PubMed]
178. Abdureyim, A.; Abliz, M.; Sultan, A.; Eshbakova, K.A. Phenolic compounds from the flowers of Coreopsis
tinctoria. Chem. Nat. Compd. 2013, 48, 1085–1086. [CrossRef]
179. Ma, Z.; Zheng, S.; Han, H.; Meng, J.; Yang, X.; Zeng, S.; Zhou, H.; Jiang, H. The bioactive components of
Coreopsis tinctoria (Asteraceae) capitula: Antioxidant activity in vitro and profile in rat plasma. J. Funct. Foods
2016, 20, 575–586. [CrossRef]
180. Chen, L.X.; Hu, D.J.; Lam, S.C.; Ge, L.; Wu, D.; Zhao, J.; Long, Z.R.; Yang, W.J.; Fan, B.; Li, S.P.
Comparison of antioxidant activities of different parts from snow chrysanthemum (Coreopsis tinctoria
Nutt.) and identification of their natural antioxidants using high performance liquid chromatography
coupled with diode array detection and mass spectrometry and 2,20-azinobis(3-ethylbenzthiazoline-sulfonic
acid)diammonium salt-based assay. J. Chromatogr. A 2016, 1428, 134–142. [PubMed]
181. Deng, Y.; Lam, S.-C.; Zhao, J.; Li, S.-P. Quantitative analysis of flavonoids and phenolic acid in Coreopsis
tinctoria Nutt. by capillary zone electrophoresis. Electrophoresis 2017, 38, 2654–2661. [CrossRef] [PubMed]
182. Yang, Y.; Sun, X.; Liu, J.; Kang, L.; Chen, S.; Ma, B.; Guo, B. Quantitative and qualitative analysis of
flavonoids and phenolic acids in snow chrysanthemum (Coreopsis tinctoria Nutt.) by HPLC-DAD and
UPLC-ESI-QTOF-MS. Molecules 2016, 21, 1307. [CrossRef] [PubMed]
183. Zalaru, C.; Cri¸san, C.C.; C ˇ alinescu, I.; Moldovan, Z.; ¸T ˇ ârcomnicu, I.; Litescu, S.C.; Tatia, R.; Moldovan, L.;
Boda, D.; Iovu, M. Polyphenols in Coreopsis tinctoria Nutt. fruits and the plant extracts antioxidant capacity
evaluation. Cent. Eur. J. Chem. 2014, 12, 858–867. [CrossRef]
184. Wang, T.; Xi, M.; Guo, Q.; Wang, L.; Shen, Z. Chemical components and antioxidant activity of volatile oil of
a Compositae tea (Coreopsis tinctoria Nutt.) from Mt. Kunlun. Ind. Crops Prod. 2015, 67, 318–323. [CrossRef]
185. Hostettmann, K.; Hostettmann-Kaldas, M.; Nakanishi, K. Molluscicidal saponins from Cornus florida L. Helv.
Chim. Acta 1978, 61, 1990–1995. [CrossRef]
186. Robins, R.J.; Abraham, T.W.; Parr, A.J.; Eagles, J.; Walton, N.J. The biosynthesis of tropane alkaloids in Datura
stramonium: The identity of the intermediates between N-methylpyrrolinium salt and tropinone. J. Am.
Chem. Soc. 1997, 119, 10929–10934. [CrossRef]
187. Monforte-González, M.; Ayora-Talavera, T.; Maldonado-Mendoza, E.; Loyola-Vargas, V.M. Quantitative
analysis of serpentine and ajmalicine in plant tissues of Catharanthus roseus and hyoscyamine
and scopolamine in root tissues of Datura stramonium by thin layer chromatography-densitometry.
Phytochem. Anal. 1992, 3, 117–121. [CrossRef]
188. Lanfranchi, D.A.; Tomi, F.; Casanova, J. Enantiomeric differentiation of atropine/hyoscyamine by 13C NMR
spectroscopy and its application to Datura stramonium extract. Phytochem. Anal. 2010, 21, 597–601. [CrossRef]
[PubMed]
189. Mroczek, T.; Głowniak, K.; Kowalska, J. Solid-liquid extraction and cation-exchange solid-phase extraction
using a mixed-mode polymeric sorbent of Datura and related alkaloids. J. Chromatogr. A 2006, 1107, 9–18.
[CrossRef] [PubMed]
190. Fallas, A.L.; Thomson, R.H. Ebenaceae extractives. Part III. Binaphthaquinones from Diospyros species. J.
Chem. Soc. C Org. 1968, 1968, 2279–2282. [CrossRef]
191. Rashed, K.; Ciri´c, A.; Glamoˇclija, J.; Sokovi´c, M. Antibacterial and antifungal activities of methanol extract ´
and phenolic compounds from Diospyros virginiana L. Ind. Crops Prod. 2014, 59, 210–215. [CrossRef]
192. Wang, X.; Habib, E.; León, F.; Radwan, M.M.; Tabanca, N.; Gao, J.; Wedge, D.E.; Cutler, S.J. Antifungal
metabolites from the roots of Diospyros virginiana by overpressure layer chromatography. Chem. Biodivers.
2011, 8, 2331–2340. [CrossRef] [PubMed]
193. Kiss, A.; Kowalski, J.; Melzig, M.F. Compounds from Epilobium angustifolium inhibit the specific
metallopeptidases ACE, NEP and APN. Planta Med. 2004, 70, 919–923. [CrossRef] [PubMed]
194. Kiss, A.; Kowalski, J.; Melzig, M.F. Effect of Epilobium angustifolium L. extracts and polyphenols on cell
proliferation and neutral endopeptidase activity in selected cell lines. Pharmazie 2006, 61, 66–69. [PubMed]
195. Ramstead, A.G.; Schepetkin, I.A.; Quinn, M.T.; Jutila, M.A. Oenothein B, a cyclic dimeric ellagitannin
isolated from Epilobium angustifolium, enhances IFNγ production by lymphocytes. PLoS ONE 2012, 7, e50546.
[CrossRef] [PubMed]
196. Baert, N.; Karonen, M.; Salminen, J.P. Isolation, characterisation and quantification of the main oligomeric
macrocyclic ellagitannins in Epilobium angustifolium by ultra-high performance chromatography with diode
array detection and electrospray tandem mass spectrometry. J. Chromatogr. A 2015, 1419, 26–36. [CrossRef]
[PubMed]
197. Baert, N.; Kim, J.; Karonen, M.; Salminen, J.P. Inter-population and inter-organ distribution of the main
polyphenolic compounds of Epilobium angustifolium. Phytochemistry 2017, 134, 54–63. [CrossRef] [PubMed]
198. Park, B.-J.; Tomohiko, M. Feruloyl, caffeoyl, and flavonol glucosides from Equisetum hyemale. Chem. Nat.
Compd. 2011, 47, 363–365. [CrossRef]
199. Jin, M.; Zhang, C.; Zheng, T.; Yao, D.; Shen, L.; Luo, J.; Jiang, Z.; Ma, J.; Jin, X.-J.; Cui, J.; et al. A new
phenyl glycoside from the aerial parts of Equisetum hyemale. Nat. Prod. Res. 2014, 28, 1813–1818. [CrossRef]
[PubMed]
200. Price, J.I. An in vitro evaluation of the Native American ethnomedicinal plant Eryngium yuccifolium as a
treatment for snakebite envenomation. J. Intercult. Ethnopharmacol. 2016, 5, 219–225. [CrossRef] [PubMed]
201. Yarnell, E.; Abascal, K. Natural approaches to treating chronic prostatitis and chronic pelvic pain syndromes.
Altern. Complement. Ther. 2005, 11, 246–251. [CrossRef]
202. Ayoub, N.; Al-Azizi, M.; König, W.; Kubeczka, K.H. Essential oils and a novel polyacetylene from Eryngium
yuccifolium Michaux. (Apiaceae). Flavour Fragr. J. 2006, 21, 864–868. [CrossRef]
203. Zhang, Z.; Li, S.; Ownby, S.; Wang, P.; Yuan, W.; Zhang, W.; Beasley, R.S. Phenolic compounds and rare
polyhydroxylated triterpenoid saponins from Eryngium yuccifolium. Phytochemistry 2008, 69, 2070–2080.
[CrossRef] [PubMed]
204. Wang, P.; Yuan, W.; Deng, G.; Su, Z.; Li, S. Triterpenoid saponins from Eryngium yuccifolium “Kershaw Blue”.
Phytochem. Lett. 2013, 6, 306–309. [CrossRef]
205. Wang, P.; Su, Z.; Yuan, W.; Deng, G.; Li, S. Phytochemical constituents and pharmacological activities of
Eryngium L. (Apiaceae). Pharm. Crop. 2012, 3, 99–120. [CrossRef]
206. Cavallito, C.J.; Haskell, T.H. α-Methylene butyrolactone from Erythronium americanum. J. Am. Chem. Soc.
1946, 68, 2332–2334. [CrossRef] [PubMed]
207. Tsuda, Y.; Marion, L. The alkaloids of Eupatorium maculatum L. Can. J. Chem. 1963, 41, 1919–1924. [CrossRef]
208. Wiedenfeld, H.; Hösch, G.; Roeder, E.; Dingermann, T. Lycopsamine and cumambrin B from Eupatorium
maculatum. Pharmazie 2009, 64, 415–416. [PubMed]
209. Maas, M.; Hensel, A.; Da Costa, F.B.; Brun, R.; Kaiser, M.; Schmidt, T.J. An unusual dimeric guaianolide with
antiprotozoal activity and further sesquiterpene lactones from Eupatorium perfoliatum. Phytochemistry 2011,
72, 635–644. [CrossRef] [PubMed]
210. Herz, W.; Kalyanaraman, P.S.; Ramakrishnan, G.; Blount, J.F. Sesquiterpene lactones of Eupatorium perfoliatum.
J. Org. Chem. 1977, 42, 2264–2271. [CrossRef] [PubMed]
211. Habtemariam, S. Activity-guided isolation and identification of free radical-scavenging components from
ethanolic extract of boneset (leaves of Eupatorium perfoliatum). Nat. Prod. Commun. 2008, 3, 1317–1320.
212. Maas, M.; Deters, A.M.; Hensel, A. Anti-inflammatory activity of Eupatorium perfoliatum L. extracts, eupafolin,
and dimeric guaianolide via iNOS inhibitory activity and modulation of inflammation-related cytokines and
chemokines. J. Ethnopharmacol. 2011, 137, 371–381. [CrossRef] [PubMed]
213. Maas, M.; Petereit, F.; Hensel, A. Caffeic acid derivatives from Eupatorium perfoliatum L. Molecules 2009, 14,
36–45. [CrossRef] [PubMed]
214. Herz, W. Chemistry of the Eupatoriinae. Biochem. Syst. Ecol. 2001, 29, 1115–1137. [CrossRef]
215. Hensel, A.; Maas, M.; Sendker, J.; Lechtenberg, M.; Petereit, F.; Deters, A.; Schmidt, T.; Stark, T. Eupatorium
perfoliatum L.: Phytochemistry, traditional use and current applications. J. Ethnopharmacol. 2011, 138, 641–651.
[CrossRef] [PubMed]
216. Lewis, N.G.; Inciong, M.E.J.; Ohashi, H.; Towers, G.H.N.; Yamamoto, E. Exclusive accumulation of Z-isomers
of monolignols and their glucosides in bark of Fagus grandifolia. Phytochemistry 1988, 27, 2119–2121.
[CrossRef]
217. Stout, G.H.; Balkenhol, W.J. Xanthones of the Gentianaceae-I: Frasera caroliniensis. Tetrahedron 1969, 25,
1947–1960. [CrossRef]
218. Aberham, A.; Pieri, V.; Croom, E.M.; Ellmerer, E.; Stuppner, H. Analysis of iridoids, secoiridoids and
xanthones in Centaurium erythraea, Frasera caroliniensis and Gentiana lutea using LC-MS and RP-HPLC. J.
Pharm. Biomed. Anal. 2011, 54, 517–525. [CrossRef] [PubMed]
219. Eyles, A.; Jones, W.; Riedl, K.; Cipollini, D.; Schwartz, S.; Chan, K.; Herms, D.A.; Bonello, P. Comparative
phloem chemistry of Manchurian (Fraxinus mandshurica) and two North American ash species (Fraxinus
americana and Fraxinus pennsylvanica). J. Chem. Ecol. 2007, 33, 1430–1448. [CrossRef] [PubMed]
220. Takenaka, Y.; Tanahashi, T.; Shintaku, M.; Sakai, T.; Nagakura, N. Parida Secoiridoid glucosides from Fraxinus
americana. Phytochemistry 2000, 55, 275–284. [CrossRef]
221. Aybek, A.; Zhou, J.; Malik, A.; Umar, S.; Xiao, Z. Catechins and proanthocyanidins from seeds of Fraxinus
americana. Chem. Nat. Compd. 2015, 51, 565–567. [CrossRef]
222. Gallardo, A.; Picollo, M.I.; González-Audino, P.; Mougabure-Cueto, G. Insecticidal activity of individual and
mixed monoterpenoids of Geranium essential oil against Pediculus humanus capitis (Phthiraptera: Pediculidae).
J. Med. Entomol. 2012, 49, 332–335. [CrossRef] [PubMed]
223. Sánchez-Tena, S.; Fernández-Cachón, M.L.; Carreras, A.; Mateos-Martín, M.L.; Costoya, N.; Moyer, M.P.;
Nuñez, M.J.; Torres, J.L.; Cascante, M. Hamamelitannin from witch hazel (Hamamelis virginiana) displays
specific cytotoxic activity against colon cancer cells. J. Nat. Prod. 2012, 75, 26–33. [CrossRef] [PubMed]
224. Duckstein, S.M.; Stintzing, F.C. Investigation on the phenolic constituents in Hamamelis virginiana leaves by
HPLC-DAD and LC-MS/MS. Anal. Bioanal. Chem. 2011, 401, 677–688. [CrossRef] [PubMed]
225. Dauer, A.; Rimpler, H.; Hensel, A. Polymeric proanthocyanidins from the bark of Hamamelis virginiana. Planta
Med. 2003, 69, 89–91. [CrossRef] [PubMed]
226. Touriño, S.; Lizárraga, D.; Carreras, A.; Lorenzo, S.; Ugartondo, V.; Mitjans, M.; Vinardell, M.P.; Julía, L.;
Cascante, M.; Torres, J.L. Highly galloylated tannin fractions from witch hazel (Hamamelis virginiana) bark:
Electron transfer capacity, in vitro antioxidant activity, and effects on skin-related cells. Chem. Res. Toxicol.
2008, 21, 696–704. [CrossRef] [PubMed]
227. Hartisch, C.; Kolodziej, H. Galloylhamameloses and proanthocyanidins from Hamamelis virginiana.
Phytochemistry 1996, 42, 191–198. [CrossRef]
228. Lucas, R.A.; Smith, R.G.; Dorfman, L. The isolation of dihydromexicanin E from Helenium autumnale L. J.
Org. Chem. 1964, 29, 2101. [CrossRef]
229. Herz, W.; Subramaniam, P.S.; Dennis, N. Constituents of Helenium species. XXIII. Stereochemistry of flexuosin
A and related compounds. J. Org. Chem. 1969, 34, 2915–2917. [CrossRef]
230. Herz, W.; de Vivar, A.R.; Romo, J.; Viswanathan, N. Constituents of Helenium species. XIII. The structure of
helenalin and mexicanin A. J. Am. Chem. Soc. 1963, 85, 19–26. [CrossRef]
231. Herz, W.; Subramaniam, P.S. Pseudoguianolides in Helenium autumnale from Pennsylvania. Phytochemistry
1972, 11, 1101–1103. [CrossRef]
232. Lee, K.-H.; Meck, R.; Piantadosi, C.; Huang, E.-S. Antitumor agents. 4. Cytotoxicity and in vivo activity of
helenalin esters and related derivatives. J. Med. Chem. 1973, 16, 299–301. [CrossRef] [PubMed]
233. Furukawa, H.; Lee, K.-H.; Shingu, T.; Meck, R.; Piantadosi, C. Carolenin and carolenalin, two new
guaianolides in Helenium autumnale L. from North Carolina. J. Org. Chem. 1973, 38, 1722–1725. [CrossRef]
[PubMed]
234. Pettit, G.R.; Budzinski, J.C.; Cragg, G.M.; Brown, P.; Johnston, L.D. Antineoplastic agents. 34. Helenium
autumnale L. J. Med. Chem. 1974, 17, 1013–1016. [CrossRef] [PubMed]
235. Kozuka, M.; Lee, K.-H.; McPhail, A.T.; Onan, K.D. Structure and absolute stereochemistry of
dihydroflorilenalin, a new sesquiterpene lactone from Florida Helenium autumnale L. Chem. Pharm. Bull.
1975, 23, 1895–1897. [CrossRef]
236. Furukawa, H.; Itoigawa, M.; Kumagai, N.; Ito, K.; McPhail, A.T.; Onan, K.D. Isolation and structure
determination of 4-O-tigloyl-11,13-dihydroautumnolide, a new sesquiterpene lactone from North Carolina
Helenium autumnale L. Chem. Pharm. Bull. 1978, 25, 1335–1337. [CrossRef]
237. Gentry, E.J.; Jampani, H.B.; Keshavarz-Shokri, A.; Morton, M.D.; Vander Velde, D.; Telikepalli, H.;
Mitscher, L.A.; Shawar, R.; Humble, D.; Baker, W. Antitubercular natural products: Berberine from the roots
of commercial Hydrastis canadensis powder. Isolation of inactive 8-oxotetrahydrothalifendine, canadine,
β-hydrastine, and two new quinic acid esters, hycandinic acid esters-1 and -2. J. Nat. Prod. 1998, 61,
1187–1193. [CrossRef] [PubMed]
238. Scazzocchio, F.; Cometa, M.F.; Tomassini, L.; Palmery, M. Antibacterial activity of Hydrastis canadensis extract
and its major isolated alkaloids. Planta Med. 2001, 67, 561–564. [CrossRef] [PubMed]
239. Chadwick, L.R.; Wu, C.D.; Kinghorn, A.D. Isolation of alkaloids from goldenseal (Hydrastis canadensis
rhizomes) using pH-zone refining countercurrent chromatography. J. Liq. Chromatogr. Relat. Technol. 2001, 24,
2445–2453. [CrossRef]
240. Le, P.M.; McCooeye, M.; Windust, A. Characterization of the alkaloids in goldenseal (Hydrastis canadensis)
root by high resolution Orbitrap LC-MSn. Anal. Bioanal. Chem. 2013, 405, 4487–4498. [CrossRef] [PubMed]
241. Leyte-Lugo, M.; Britton, E.R.; Foil, D.H.; Brown, A.R.; Todd, D.A.; Rivera-Chávez, J.; Oberlies, N.H.;
Cech, N.B. Secondary metabolites from the leaves of the medicinal plant goldenseal (Hydrastis canadensis).
Phytochem. Lett. 2017, 20, 54–60. [CrossRef] [PubMed]
242. Junio, H.A.; Sy-Cordero, A.A.; Ettefagh, K.A.; Burns, J.T.; Micko, K.T.; Graf, T.N.; Richter, S.J.; Cannon, R.E.;
Oberlies, N.H.; Cech, N.B. Synergy-directed fractionation of botanical medicines: A case study with
goldenseal (Hydrastis canadensis). J. Nat. Prod. 2011, 74, 1621–1629. [CrossRef] [PubMed]
243. Babka, H.L.; Hillwig, M.L.; Price, J.; Maury, W.; Harslan, H.; Wu, L.; Wurtele, E.S. Hypericum gentianoides
produces bioactive compounds in schizogenously formed glands. Microsc. Microanal. 2010, 16, 1160–1161.
244. Crispin, M.C.; Hur, M.; Park, T.; Kim, Y.H.; Wurtele, E.S. Identification and biosynthesis of
acylphloroglucinols in Hypericum gentianoides. Physiol. Plant. 2013, 148, 354–370. [CrossRef] [PubMed]
245. Hillwig, M.L.; Hammer, K.D.P.; Birt, D.F.; Wurtele, E.S. Characterizing the metabolic fingerprint and
anti-inflammatory activity of Hypericum gentianoides. J. Agric. Food Chem. 2008, 56, 4359–4366. [CrossRef]
[PubMed]
246. Christian, O.E.; McLean, S.; Reynolds, W.F.; Jacobs, H. Prenylated benzophenones from Hypericum hypericoides.
Nat. Prod. Commun. 2008, 3, 1781–1786.
247. Dictionary of Natural Products Dictionary of Natural Products on DVD. J. Antibiot. 1994, 48, 261–266.
248. Gupta, S.R.; Ravindranath, B.; Seshadri, T.R. Polyphenols of Juglans nigra. Phytochemistry 1972, 11, 2634–2636.
[CrossRef]
249. Binder, R.G.; Benson, M.E.; Flath, R.A. Eight 1,4-naphthoquinones from Juglans. Phytochemistry 1989, 28,
2799–2801. [CrossRef]
250. Lal, C.; Raja, A.S.M.; Pareek, P.K.; Shakyawar, D.B.; Sharma, K.K.; Sharma, M.C. Juglans nigra: Chemical
constitution and its application on Pashmina (Cashmere) fabric as a dye. J. Nat. Prod. Plant Resour. 2011, 1,
13–19.
251. Paudel, P.; Satyal, P.; Dosoky, N.S.; Maharjan, S.; Setzer, W.N. Juglans regia and J. nigra, two trees important in
traditional medicine: A comparison of leaf essential oil compositions and biological activities. Nat. Prod.
Commun. 2013, 8, 1481–1486. [PubMed]
252. Jin, D.-Z.; Min, Z.-D.; Chiou, G.C.Y.; Iinuma, M.; Tanaka, T. Two p-coumaroyl glycerides from Juncus effusus.
Phytochemistry 1996, 41, 545–547.
253. Dellagreca, M.; Fiorentino, A.; Monaco, P.; Previtera, L.; Sorrentino, M. Antialgal phenylpropane glycerides
from Juncus effusus. Nat. Prod. Lett. 1998, 12, 263–270. [CrossRef]
254. Della Greca, M.; Fiorentino, A.; Molinaro, A.; Monaco, P.; Previtera, L. A bioactive dihydrodibenzoxepin
from Juncus effusus. Phytochemistry 1993, 34, 1182–1184. [CrossRef]
255. Corsaro, M.M.; della Greca, M.; Fiorentino, A.; Monaco, P.; Previtera, L. Cycloartane glucosides from Juncus
effusus. Phytochemistry 1994, 37, 515–519. [CrossRef]
256. Della Greca, M.; Fiorentino, A.; Monaco, P.; Previtera, L. Cycloartane triterpenes from Juncus effusus.
Phytochemistry 1994, 35, 1017–1022. [CrossRef]
257. Della Greca, M.; Fiorentino, A.; Monaco, P.; Previtera, L. Juncoside I, a new cycloartanelactone glucoside
from Juncus effusus. Nat. Prod. Lett. 1994, 4, 183–188. [CrossRef]
258. Su, X.-H.; Yuan, Z.-P.; Li, C.-Y.; Zhong, Y.-J.; Du, H.-J.; Wen, Y.-Y.; Li, Y.-F.; Liang, B. Phenanthrenes from
Juncus effusus. Planta Med. 2013, 79, 1447–1452. [CrossRef] [PubMed]
259. Hanawa, F.; Okamoto, M.; Towers, G.H. Antimicrobial DNA-binding photosensitizers from the common
rush, Juncus effusus. Photochem. Photobiol. 2002, 76, 51–56. [CrossRef]
260. della Greca, M.; Fiorentino, A.; Mangoni, L.; Molinaro, A.; Monaco, P.; Previtera, L.
9,10-Dihydrophenanthrene metabolites from Juncus effusus L. Tetrahedron Lett. 1992, 33, 5257–5260.
[CrossRef]
261. Della Greca, M.; Fiorentino, A.; Mangoni, L.; Molinaro, A.; Monaco, P.; Previtera, L. Cytotoxic
9,10-dihydrophenanthrenes from Juncus effusus L. Tetrahedron 1993, 49, 3425–3432. [CrossRef]
262. DellaGreca, M.; Monaco, P.; Previtera, L.; Zarrelli, A.; Pollio, A.; Pinto, G.; Fiorentino, A. Minor bioactive
dihydrophenanthrenes from Juncus effusus. J. Nat. Prod. 1997, 60, 1265–1268. [CrossRef]
263. Della Greca, M.; Fiorentino, A.; Previtera, L.; Zarrelli, A. Effusides I–V: 9,10-Dihydrophenanthrene glucosides
from Juncus effusus. Phytochemistry 1995, 40, 533–535. [CrossRef]
264. Wang, Y.-G.; Wang, Y.-L.; Zhai, H.-F.; Liao, Y.-J.; Zhang, B.; Huang, J.-M. Phenanthrenes from Juncus effusus
with anxiolytic and sedative activities. Nat. Prod. Res. 2012, 26, 1234–1239. [CrossRef] [PubMed]
265. Yang, G.Z.; Li, H.X.; Song, F.J.; Chen, Y. Diterpenoid and phenolic compounds from Juncus effusus L. Helv.
Chim. Acta 2007, 90, 1289–1295. [CrossRef]
266. Shima, K.; Toyota, M.; Asakawa, Y. Phenanthrene derivatives from the medullae of Juncus effusus.
Phytochemistry 1991, 30, 3149–3151. [CrossRef]
267. Ishiuchi, K.; Kosuge, Y.; Hamagami, H.; Ozaki, M.; Ishige, K.; Ito, Y.; Kitanaka, S. Chemical constituents
isolated from Juncus effusus induce cytotoxicity in HT22 cells. J. Nat. Med. 2015, 69, 421–426. [CrossRef]
[PubMed]
268. Della Greca, M.; Fiorentino, A.; Monaco, P.; Previtera, L.; Zarrelli, A. Tetrahydropyrene glucosides from
Juncus effusus. Nat. Prod. Lett. 1995, 7, 85–92. [CrossRef]
269. Ma, W.; Liu, F.; Ding, Y.Y.; Zhang, Y.; Li, N. Four new phenanthrenoid dimers from Juncus effusus L. with
cytotoxic and anti-inflammatory activities. Fitoterapia 2015, 105, 83–88. [CrossRef] [PubMed]
270. Ma, W.; Zhang, Y.; Ding, Y.Y.; Liu, F.; Li, N. Cytotoxic and anti-inflammatory activities of phenanthrenes
from the medullae of Juncus effusus L. Arch. Pharm. Res. 2016, 39, 154–160. [CrossRef] [PubMed]
271. Liao, Y.J.; Zhai, H.F.; Zhang, B.; Duan, T.X.; Huang, J.M. Anxiolytic and sedative effects of dehydroeffusol
from Juncus effusus in mice. Planta Med. 2011, 77, 416–420. [CrossRef] [PubMed]
272. Singhuber, J.; Baburin, I.; Khom, S.; Zehl, M.; Urban, E.; Hering, S.; Kopp, B. GABAA Receptor modulators
from the Chinese herbal drug junci medulla—The pith of Juncus effusus. Planta Med. 2012, 78, 455–458.
[CrossRef] [PubMed]
273. Stewart, C.D.; Jones, C.D.; Setzer, W.N. Essential oil compositions of Juniperus virginiana and Pinus virginiana,
two important trees in Cherokee traditional medicine. Am. J. Essent. Oils Nat. Prod. 2014, 2, 17–24.
274. Adams, R.P. Cedar wood oil—Analyses and properties. In Essential Oils and Waxes; Linskens, H.F.,
Jackson, J.F., Eds.; Springer: Berlin, Germany, 1991; pp. 159–173.
275. Tumen, I.; Süntar, I.; Eller, F.J.; Kele¸s, H.; Akkol, E.K. Topical wound-healing effects and phytochemical
composition of heartwood essential oils of Juniperus virginiana L., Juniperus occidentalis Hook., and Juniperus
ashei J. Buchholz. J. Med. Food 2013, 16, 48–55. [CrossRef] [PubMed]
276. Renouard, S.; Lopez, T.; Hendrawati, O.; Dupre, P.; Doussot, J.; Falguieres, A.; Ferroud, C.; Hagege, D.;
Lamblin, F.; Laine, E.; et al. Podophyllotoxin and deoxypodophyllotoxin in Juniperus bermudiana and 12
other Juniperus species: Optimization of extraction, method validation, and quantification. J. Agric. Food
Chem. 2011, 59, 8101–8107. [CrossRef] [PubMed]
277. Michalska, K.; Szneler, E.; Kisiel, W. Sesquiterpene lactones from Lactuca canadensis and their chemotaxonomic
significance. Phytochemistry 2013, 90, 90–94. [CrossRef] [PubMed]
278. Kagan, J. The flavonoid pigments of Liatris spicata. Phytochemistry 1968, 7, 1205–1207. [CrossRef]
279. Karlsson, K.; Wahlberg, I.; Enzell, C.R. Volatile constituents of the Liatris species, L. spicata, L. elegans and L.
gracilis. Acta Chem. Scand. 1973, 27, 1613–1621. [CrossRef] [PubMed]
280. Herz, W.; Poplawski, J.; Sharma, R.P. New guaianolides from Liatris species. J. Org. Chem. 1975, 40, 199–206.
[CrossRef]
281. Ezzat, M.I.; Ezzat, S.M.; El Deeb, K.S.; El Fishawy, M. In vitro cytotoxic activity of the ethanol extract and
isolated compounds from the corms of Liatris spicata (L.) Willd on HepG2. Nat. Prod. Res. 2017, 31, 1325–1328.
[CrossRef] [PubMed]
282. Setzer, W.N. Chemical composition of the leaf essential oil of Lindera benzoin growing in North Alabama. Am.
J. Essent. Oils Nat. Prod. 2016, 4, 1–3.
283. Tucker, A.O.; Maciarello, M.J.; Burbage, P.W.; Sturtz, G. Spicebush [Lindera benzoin (L.) Blume var. benzoin,
Lauraceae]: A tea, spice, and medicine. Econ. Bot. 1994, 48, 333–336.
284. Anderson, J.E.; Ma, W.; Smith, D.L.; Chang, C.-J.; McLaughlin, J.L. Biologically active γ-lactones and
methylketoalkenes from Lindera benzoin. J. Nat. Prod. 1992, 55, 71–83. [CrossRef] [PubMed]
285. Martin, E.; Duke, J.; Pelkki, M.; Clausen, E.C.; Carrier, D.J. Sweetgum (Liquidambar styraciflua L.): Extraction of
shikimic acid coupled to dilute acid pretreatment. Appl. Biochem. Biotechnol. 2010, 162, 1660–1668. [CrossRef]
[PubMed]
286. Sakai, K.; Fukuda, Y.; Matsunaga, S.; Tanaka, R.; Yamori, T. New cytotoxic oleanane-type triterpenoids from
the cones of Liquidamber styraciflua. J. Nat. Prod. 2004, 67, 1088–1093. [CrossRef] [PubMed]
287. Rajan, K.; Nelson, A.; Adams, J.P.; Carrier, D.J. Phytochemical recovery for valorization of loblolly pine and
sweetgum bark residues. ACS Sustain. Chem. Eng. 2017, 5, 4258–4266. [CrossRef]
288. Fukuda, Y.; Yamada, T.; Wada, S.I.; Sakai, K.; Matsunaga, S.; Tanaka, R. Lupane and oleanane triterpenoids
from the cones of Liquidamber styraciflua. J. Nat. Prod. 2006, 69, 142–144. [CrossRef] [PubMed]
289. Eid, H.H.; Labib, R.M.; Hamid, N.S.A.; Hamed, M.A.; Ross, S.A. Hepatoprotective and antioxidant
polyphenols from a standardized methanolic extract of the leaves of Liquidambar styraciflua L. Bull. Fac.
Pharm. Cairo Univ. 2015, 53, 117–127. [CrossRef]
290. Rashed, K.N.Z.; Sucupira, A.C.C.; Ferreira, P.M.P.; Feitosa, C.M. Phytoconstituents and evaluation of
acetylcholinesterase inhibition by methanol extract of Liquidambar styraciflua (L.) aerial parts. J. Appl. Pharm.
2014, 6, 143–152. [CrossRef]
291. El-Readi, M.Z.; Eid, H.H.; Ashour, M.L.; Eid, S.Y.; Labib, R.M.; Sporer, F.; Wink, M. Variations of the chemical
composition and bioactivity of essential oils from leaves and stems of Liquidambar styraciflua (Altingiaceae).
J. Pharm. Pharmacol. 2013, 65, 1653–1663. [CrossRef] [PubMed]
292. Chen, C.-L.; Chang, H.-M. Lignans and aporphine alkaloids in bark of Liriodendron tulipifera. Phytochemistry
1978, 17, 779–782. [CrossRef]
293. Graziose, R.; Rathinasabapathy, T.; Lategan, C.; Poulev, A.; Smith, P.J.; Grace, M.; Lila, M.A.; Raskin, I.
Antiplasmodial activity of aporphine alkaloids and sesquiterpene lactones from Liriodendron tulipifera L. J.
Ethnopharmacol. 2011, 133, 26–30. [CrossRef] [PubMed]
294. Kang, Y.-F.; Liu, C.-M.; Kao, C.-L.; Chen, C.-Y. Antioxidant and anticancer constituents from the leaves of
Liriodendron tulipifera. Molecules 2014, 19, 4235–4245. [CrossRef] [PubMed]
295. Doskotch, R.W.; Wilton, J.H.; Harraz, F.M.; Fairchild, E.H.; Huang, C.T.; El-Feraly, F.S. Six additional
sesquiterpene lactones from Liriodendron tulipifera. J. Nat. Prod. 1983, 46, 923–929. [CrossRef]
296. Jeong, E.J.; Kim, N.-H.; Heo, J.-D.; Lee, K.Y.; Rho, J.-R.; Kim, Y.C.; Sung, S.H. Antifibrotic compounds from
Liriodendron tulipifera attenuating HSC-T6 proliferation and TNF-α production in RAW264.7 cells. Biol.
Pharm. Bull. 2015, 38, 228–234. [CrossRef] [PubMed]
297. Doskotch, R.W.; El-Feraly, F.S. The structure of tulipinolide and epitulipinolide. Cytotoxic sesquiterpenes
from Liriodendron tulipifera L. J. Org. Chem. 1970, 35, 1928–1936. [CrossRef] [PubMed]
298. Miller, S.L.; Villanueva, H.E.; Palazzo, M.C.; Wright, B.S.; Setzer, W.N. Seasonal variation and bioactivity in
the leaf oil of Liriodendron tulipifera growing in Huntsville, Alabama. Nat. Prod. Commun. 2009, 4, 839–843.
[PubMed]
299. Smith, A.L.; Campbell, C.L.; Walker, D.B.; Hanover, J.W.; Miller, R.O. Geographic variation in the essential
oil monoterpenes of Liriodendron tulipifera L. Biochem. Syst. Ecol. 1988, 16, 627–630. [CrossRef]
300. Brown, D.P.; Rogers, D.T.; Pomerleau, F.; Siripurapu, K.B.; Kulshrestha, M.; Gerhardt, G.A.; Littleton, J.M.
Novel multifunctional pharmacology of lobinaline, the major alkaloid from Lobelia cardinalis. Fitoterapia 2016,
111, 109–123. [CrossRef] [PubMed]
301. Yamanaka, M.; Ishibashi, K.; Shimomura, K.; Ishimaru, K. Polyacetylene glucosides in hairy root cultures of
Lobelia cardinalis. Phytochemistry 1996, 41, 183–185. [CrossRef]
302. Vodopivec, B.M.; Wang, J.; Møller, A.L.; Krake, J.; Lund, T.; Hansen, P.E.; Nielsen, S.L. Differences in the
structure of anthocyanins from the two amphibious plants, Lobelia cardinalis and Nesaea crassicaulis. Nat. Prod.
Res. 2013, 27, 655–664. [CrossRef] [PubMed]
303. Bálványos, I.; Kursinszki, L.; Bányai, P.; Szöke, É. Analysis of polyacetylenes by HPLC in hairy root cultures
of Lobelia inflata cultivated in bioreactor. Chromatographia 2004, 60, S235–S238. [CrossRef]
304. Kursinszki, L.; Ludányi, K.; Szöke, É. LC-DAD and LC-MS-MS analysis of piperidine alkaloids of Lobelia
inflata L. (in vitro and in vivo). Chromatographia 2008, 68, S27–S33. [CrossRef]
305. Kursinszki, L.; Szöke, É. HPLC-ESI-MS/MS of brain neurotransmitter modulator lobeline and related
piperidine alkaloids in Lobelia inflata L. J. Mass Spectrom. 2015, 50, 727–733. [CrossRef] [PubMed]
306. Resting, J.R.; Tolderlund, I.-L.; Pedersen, A.F.; Witt, M.; Jaroszewski, J.W.; Staerk, D. Piperidine and
tetrahydropyridine alkaloids from Lobelia siphilitica and Hippobroma longiflora. J. Nat. Prod. 2009, 72, 312–315.
[CrossRef] [PubMed]
307. Bucar, F.; Kartnig, T. Flavone glucuronides of Lycopus virginicus. Planta Med. 1995, 61, 378–380. [CrossRef]
[PubMed]
308. Doskotch, R.W.; Flom, M.S. Acuminatin, a new bis-phenylpropide from Magnolia acuminata L. Tetrahedron
1972, 28, 4711–4717. [CrossRef]
309. Flom, M.S. Part I. The Isolation and Characterization of Alkaloids of Caulophyllum thalictroides (L.) Michx.
Part II. The Isolation and Characterization of Alkaloid and Neutral Principles of Magnolia acuminata L. Ph.D.
Thesis, The Ohio State University, Columbus, OH, USA, 1971.
310. Furmanowa, M.; Jozefowicz, J. Alkaloids as taxonomic markers in some species of Magnolia L. and
Liriodendron L. Acta Soc. Bot. Pol. 1980, 49, 527–535. [CrossRef]
311. Manske, R.H.F. An alkaloid from Menispermum canadense L. Can. J. Res. 1943, 21b, 17–20. [CrossRef]
312. Knapp, J.E. The Isolation and Chemical Characterization of Alkaloids from Menispermum canadense L. Ph.D.
Thesis, The Ohio State University, Columbus, OH, USA, 1969.
313. Carnat, A.P.; Lamaison, J.L.; Rémery, A. Composition of leaf and flower essential oil from Monarda didyma L.
cultivated in France. Flavour Fragr. J. 1991, 6, 79–80. [CrossRef]
314. Tognolini, M.; Barocelli, E.; Ballabeni, V.; Bruni, R.; Bianchi, A.; Chiavarini, M.; Impicciatore, M. Comparative
screening of plant essential oils: Phenylpropanoid moiety as basic core for antiplatelet activity. Life Sci. 2006,
78, 1419–1432. [CrossRef] [PubMed]
315. Fraternale, D.; Giamperi, L.; Bucchini, A.; Ricci, D.; Epifano, F.; Burini, G.; Curini, M. Chemical composition,
antifungal and in vitro antioxidant properties of Monarda didyma L. essential oil. J. Essent. Oil Res. 2006, 18,
581–585. [CrossRef]
316. Gwinn, K.D.; Ownley, B.H.; Greene, S.E.; Clark, M.M.; Taylor, C.L.; Springfield, T.N.; Trently, D.J.; Green, J.F.;
Reed, A.; Hamilton, S.L. Role of essential oils in control of Rhizoctonia damping-off in tomato with bioactive
Monarda herbage. Phytopathology 2010, 100, 493–501. [CrossRef] [PubMed]
317. Adebayo, O.; Bélanger, A.; Khanizadeh, S. Variable inhibitory activities of essential oils of three Monarda
species on the growth of Botrytis cinerea. Can. J. Plant Sci. 2013, 93, 987–995. [CrossRef]
318. Mattarelli, P.; Epifano, F.; Minardi, P.; Di Vito, M.; Modesto, M.; Barbanti, L.; Bellardi, M.G. Chemical
composition and antimicrobial activity of essential oils from aerial parts of Monarda didyma and Monarda
fistulosa cultivated in Italy. J. Essent. Oil-Bear. Plants 2017, 20, 76–86. [CrossRef]
319. Ricci, D.; Epifano, F.; Fraternale, D. The essential oil of Monarda didyma L. (Lamiaceae) exerts phytotoxic
activity in vitro against various weed seeds. Molecules 2017, 22, 222. [CrossRef] [PubMed]
320. Savickiene, N.; Dagilyt ˙ e, A.; Barsteigien ˙ e, Z.; Kazlauskas, S.; Vaiˇci ˙ unien ¯ e, J. Flavonoid ˙ u˛ analize raudonosios ˙
monardos (Monarda didyma L.) žieduose ir lapuose. Medicina 2002, 38, 1119–1122. [PubMed]
321. Mazza, G.; Chubey, B.B.; Kiehn, F. Essential oil of Monarda fistulosa L. var. menthaefolia, a potential source of
geraniol. Flavour Fragr. J. 1987, 2, 129–132. [CrossRef]
322. Contaldo, N.; Bellardi, M.G.; Cavicchi, L.; Epifano, F.; Genovese, S.; Curini, M.; Bertaccini, A. Phytochemical
effects of phytoplasma infections on essential oil of Monarda fistulosa L. Bull. Insectol. 2011, 64, S177–S178.
323. Tabanca, N.; Bernier, U.R.; Ali, A.; Wang, M.; Demirci, B.; Blythe, E.K.; Khan, S.I.; Baser, K.H.C.; Khan, I.A.
Bioassay-guided investigation of two Monarda essential oils as repellents of yellow fever mosquito Aedes
aegypti. J. Agric. Food Chem. 2013, 61, 8573–8580. [CrossRef] [PubMed]
324. Ahmad, A.; Ali, M.; Tandon, S. New oenotheralanosterol A and B: Constituents from the Oenothera biennis
roots. Chin. J. Chem. 2010, 28, 2474–2478. [CrossRef]
325. Singh, R.; Trivedi, P.; Bawankule, D.U.; Ahmad, A.; Shanker, K. HILIC quantification of oenotheralanosterol
A and B from Oenothera biennis and their suppression of IL-6 and TNF-α expression in mouse macrophages.
J. Ethnopharmacol. 2012, 141, 357–362. [CrossRef] [PubMed]
326. Shukla, Y.N.; Srivastava, A.; Kumar, S.; Kumar, S. Phytotoxic and antimicrobial constituents of Argyreia
speciosa and Oenothera biennis. J. Ethnopharmacol. 1999, 67, 241–245. [CrossRef]
327. Ahmad, A.; Singh, D.K.; Fatima, K.; Tandon, S.; Luqman, S. New constituents from the roots of Oenothera
biennis and their free radical scavenging and ferric reducing activity. Ind. Crops Prod. 2014, 58, 125–132.
[CrossRef]
328. Shukla, Y.N.; Srivastava, A.; Kumar, S. Aryl, lipid and triterpenoid constituents from Oenothera biennis. Indian
J. Chem. 1999, 38, 705–708.
329. Montserrat-de la Paz, S.; Fernández-Arche, M.A.; Ángel-Martín, M.; García-Giménez, M.D. Phytochemical
characterization of potential nutraceutical ingredients from evening primrose oil (Oenothera biennis L.).
Phytochem. Lett. 2014, 8, 158–162. [CrossRef]
330. Wettasinghe, M.; Shahidi, F.; Amarowicz, R. Identification and quantification of low molecular weight
phenolic antioxidants in seeds of evening primrose (Oenothera biennis L.). J. Agric. Food Chem. 2002, 50,
1267–1271. [CrossRef] [PubMed]
331. Zadernowski, R.; Naczk, M.; Nowak-Polakowska, H. Phenolic acids of borage (Borago officinalis L.) and
evening primrose (Oenothera biennis L.). J. Am. Oil Chem. Soc. 2002, 79, 335–338. [CrossRef]
332. Granica, S.; Czerwi´nska, M.E.; Piwowarski, J.P.; Ziaja, M.; Kiss, A.K. Chemical composition, antioxidative
and anti-inflammatory activity of extracts prepared from aerial parts of Oenothera biennis L. and Oenothera
paradoxa Hudziok obtained after seeds cultivation. J. Agric. Food Chem. 2013, 61, 801–810. [CrossRef]
[PubMed]
333. Assinewe, V.A.; Baum, B.R.; Gagnon, D.; Arnason, J.T. Phytochemistry of wild populations of Panax
quinquefolius L. (North American ginseng). J. Agric. Food Chem. 2003, 51, 4549–4553. [CrossRef] [PubMed]
334. Wang, A.; Wang, C.Z.; Wu, J.A.; Osinski, J.; Yuan, C.S. Determination of major ginsenosides in Panax
quinquefolius (American ginseng) using high-performance liquid chromatography. Phytochem. Anal. 2005, 16,
272–277. [CrossRef] [PubMed]
335. Corbit, R.M.; Ferreira, J.F.S.; Ebbs, S.D.; Murphy, L.L. Simplified extraction of ginsenosides from American
ginseng (Panax quinquefolius L.) for high-performance liquid chromatography-ultraviolet analysis. J. Agric.
Food Chem. 2005, 53, 9867–9873. [CrossRef] [PubMed]
336. Qu, C.; Bai, Y.; Jin, X.; Wang, Y.; Zhang, K.; You, J.; Zhang, H. Study on ginsenosides in different parts and
ages of Panax quinquefolius L. Food Chem. 2009, 115, 340–346. [CrossRef]
337. Christensen, L.P.; Jensen, M.; Kidmose, U. Simultaneous determination of ginsenosides and polyacetylenes
in American ginseng root (Panax quinquefolium L.) by high-performance liquid chromatography. J. Agric.
Food Chem. 2006, 54, 8995–9003. [CrossRef] [PubMed]
338. Wang, C.-Z.; Aung, H.H.; Ni, M.; Wu, J.-A.; Tong, R.; Wicks, S.; He, T.-C.; Yuan, C.-S. Red American ginseng:
Ginsenoside constituents and antiproliferative activities of heat-processed Panax quinquefolius roots. Planta
Med. 2007, 73, 669–674. [CrossRef] [PubMed]
339. Wang, Y.; Choi, H.-K.; Brinckmann, J.A.; Jiang, X.; Huang, L. Chemical analysis of Panax quinquefolius (North
American ginseng): A review. J. Chromatogr. A 2015, 1426, 1–15. [CrossRef] [PubMed]
340. Mancuso, C.; Santangelo, R. Panax ginseng and Panax quinquefolius: From pharmacology to toxicology. Food
Chem. Toxicol. 2017, 107, 362–372. [CrossRef] [PubMed]
341. Yang, W.-Z.; Hu, Y.; Wu, W.-Y.; Ye, M.; Guo, D.-A. Saponins in the genus Panax L. (Araliaceae): A systematic
review of their chemical diversity. Phytochemistry 2014, 106, 7–24. [CrossRef] [PubMed]
342. Yuan, C.-S.; Wang, C.-Z.; Wicks, S.M.; Qi, L.-W. Chemical and pharmacological studies of saponins with a
focus on American ginseng. J. Ginseng Res. 2010, 34, 160–167. [CrossRef] [PubMed]
343. Lee, T.M.; Der Marderosian, A.H. Studies on the constituents of dwarf ginseng. Phyther. Res. 1988, 2, 165–169.
[CrossRef]
344. Lui, J.H.-C.; Staba, E.J. The ginsenosides of various ginseng plants and selected products. J. Nat. Prod. 1980,
43, 340–346. [CrossRef]
345. Tanaka, T.; Iinuma, M.; Murata, H. Stilbene derivatives in the stem of Parthenocissus quinquefolia.
Phytochemistry 1998, 48, 1045–1049. [CrossRef]
346. Yang, J.B.; Wang, A.G.; Ji, T.F.; Su, Y.L. Two new oligostilbenes from the stem of Parthenocissus quinquefolia. J.
Asian Nat. Prod. Res. 2014, 16, 275–280. [CrossRef] [PubMed]
347. Chistokhodova, N.A.; Zhiviriga, I.; Nguen, C.; Miles, G.D.; Uzhegova, N.A.; Solodnikov, S.Y.
β-Amyrylhexadecanoate from Parthenocissus quinquefolia as a thrombin inhibitor. Pharm. Chem. J. 2002, 36,
245–247. [CrossRef]
348. Li, Q.; van den Heuvel, H.; Delorenzo, O.; Corthout, J.; Pieters, L.A.C.; Vlietinck, A.J.; Claeys, M. Mass
spectral characterization of C-glycosidic flavonoids isolated from a medicinal plant (Passiflora incarnata). J.
Chromatogr. B 1991, 562, 435–446.
349. Raffaelli, A.; Moneti, G.; Mercati, V.; Toja, E. Mass spectrometric characterization of flavonoids in extracts
from Passiflora incarnata. J. Chromatogr. A 1997, 777, 223–231. [CrossRef]
350. Rahman, K.; Krenn, L.; Kopp, B.; Schubert-Zsilavecz, M.; Mayer, K.K.; Kubelka, W.
Isoscoparin-2”-O-glucoside from Passiflora incarnata. Phytochemistry 1997, 45, 1093–1094. [CrossRef]
351. Chimichi, S.; Mercati, V.; Moneti, G.; Raffaelli, A.; Toja, E. Isolation and characterization of an unknown
flavonoid in dry extracts from Passiflora incarnata. Nat. Prod. Lett. 1998, 11, 225–232. [CrossRef]
352. Dhawan, K.; Dhawan, S.; Sharma, A. Passiflora: A review update. J. Ethnopharmacol. 2004, 94, 1–23. [CrossRef]
[PubMed]
353. Woo, W.S.; Kang, S.S.; Wagner, H.; Seligmann, O.; Chari, V.M. Triterpenoid saponins from the roots of
Phytolacca americana. Planta Med. 1978, 34, 87–92. [CrossRef]
354. Woo, W.S.; Kang, S.S. Phytolaccoside B: Triterpene glucoside from Phytolacca americana. Phytochemistry 1976,
15, 1315–1317. [CrossRef]
355. Suga, Y.; Maruyama, Y.; Kawanishi, S.; Shoji, J. Studies on the constituents of phytolaccaceous plants. I. On
the structures of phytolaccasaponin B, E and G from the roots of Phytolacca americana L. Chem. Pharm. Bull.
1978, 25, 520–525. [CrossRef]
356. Wang, L.; Bai, L.; Nagasawa, T.; Hasegawa, T.; Yang, X.; Sakai, J.-I.; Bai, Y.; Kataoka, T.; Oka, S.; Hirose, K.; et al.
Bioactive triterpene saponins from the roots of Phytolacca americana. J. Nat. Prod. 2008, 71, 35–40. [CrossRef]
[PubMed]
357. Seung, I.J.; Kang, J.K.; Min, K.C.; Kyung, S.K.; Lee, S.; Seon, H.A.; Seung, H.B.; Ju, H.S.; Young, S.J.; Bong, K.C.;
et al. α-Spinasterol isolated from the root of Phytolacca americana and its pharmacological property on diabetic
nephropathy. Planta Med. 2004, 70, 736–739.
358. Fleer, H.; Verspohl, E.J. Antispasmodic activity of an extract from Plantago lanceolata L. and some isolated
compounds. Phytomedicine 2007, 14, 409–415. [CrossRef] [PubMed]
359. Beara, I.N.; Lesjak, M.M.; Orˇci´c, D.Z.; Simin, N.D.; Cetojevi´c-Simin, D.D.; Božin, B.N.; Mimica-Duki´c, N.M. ˇ
Comparative analysis of phenolic profile, antioxidant, anti-inflammatory and cytotoxic activity of two
closely-related plantain species: Plantago altissima L. and Plantago lanceolata L. LWT Food Sci. Technol. 2012, 47,
64–70. [CrossRef]
360. Darrow, K.; Bowers, M.D. Phenological and population variation in iridoid glycosides of Plantago lanceolata
(Plantaginaceae). Biochem. Syst. Ecol. 1997, 25, 1–11. [CrossRef]
361. Marak, H.B.; Biere, A.; Van Damme, J.M.M. Direct and correlated responses to selection on iridoid glycosides
in Plantago lanceolata L. J. Evol. Biol. 2000, 13, 985–996. [CrossRef]
362. Gonda, S.; Tóth, L.; Gyémánt, G.; Braun, M.; Emri, T.; Vasas, G. Effect of high relative humidity on
dried Plantago lanceolata L. leaves during long-term storage: Effects on chemical composition, colour and
microbiological quality. Phytochem. Anal. 2012, 23, 88–93. [CrossRef] [PubMed]
363. Gonda, S.; Kiss, A.; Emri, T.; Batta, G.; Vasas, G. Filamentous fungi from Plantago lanceolata L. leaves:
Contribution to the pattern and stability of bioactive metabolites. Phytochemistry 2013, 86, 127–136. [CrossRef]
[PubMed]
364. Rønsted, N.; Göbel, E.; Franzyk, H.; Jensen, S.R.; Olsen, C.E. Chemotaxonomy of Plantago. Iridoid glucosides
and caffeoyl phenylethanoid glycosides. Phytochemistry 2000, 55, 337–348. [CrossRef]
365. Samuelsen, A.B. The traditional uses, chemical constituents and biological activities of Plantago major L. A
review. J. Ethnopharmacol. 2000, 71, 1–21. [CrossRef]
366. Chiang, L.C.; Chiang, W.; Chang, M.Y.; Ng, L.T.; Lin, C.C. Antiviral activity of Plantago major extracts and
related compounds in vitro. Antiv. Res. 2002, 55, 53–62. [CrossRef]
367. Zacchigna, M.; Cateni, F.; Faudale, M.; Sosa, S.; Della Loggia, R. Rapid HPLC analysis for quantitative
determination of the two isomeric triterpenic acids, oleanolic acid and ursolic acid, in Plantago major. Sci.
Pharm. 2009, 77, 79–86. [CrossRef]
368. Tarvainen, M.; Suomela, J.-P.; Kallio, H.; Yang, B. Triterpene acids in Plantago major: Identification,
quantification and comparison of different extraction methods. Chromatographia 2010, 71, 279–284. [CrossRef]
369. Kolak, U.; Boga, M.; Uru¸sak, E.A.; Ulubelen, A. Constituents of ˇ Plantago major subsp. intermedia with
antioxidant and anticholinesterase capacities. Turk. J. Chem. 2011, 35, 637–645.
370. Kartini, P.S.; Siripong, P.; Vallisuta, O. HPTLC simultaneous quantification of triterpene acids for quality
control of Plantago major L. and evaluation of their cytotoxic and antioxidant activities. Ind. Crops Prod. 2014,
60, 239–246. [CrossRef]
371. Stenholm, Å.; Göransson, U.; Bohlin, L. Bioassay-guided supercritical fluid extraction of cyclooxygenase-2
inhibiting substances in Plantago major L. Phytochem. Anal. 2013, 24, 176–183. [CrossRef] [PubMed]
372. Ibrahim, M.A.; Mansoor, A.A.; Gross, A.; Ashfaq, M.K.; Jacob, M.; Khan, S.I.; Hamann, M.T.
Methicillin-resistant Staphylococcus aureus (MRSA)-active metabolites from Platanus occidentalis (American
sycamore). J. Nat. Prod. 2009, 72, 2141–2144. [CrossRef] [PubMed]
373. Bedows, E.; Hatfield, G.M. An investigation of the antiviral activity of Podophyllum peltatum. J. Nat. Prod.
1982, 45, 725–729. [CrossRef] [PubMed]
374. Jackson, D.E.; Dewick, P.M. Aryltetralin lignans from Podophyllum hexandrum and Podophyllum peltatum.
Phytochemistry 1984, 23, 1147–1152. [CrossRef]
375. Bastos, J.K.; Burandt, C.L.; Nanayakkara, N.P.D.; Bryant, L.; McChesney, J.D. Quantitation of aryltetralin
lignans in plant parts and among different populations of Podophyllum peltatum by reversed-phase
high-performance liquid chromatography. J. Nat. Prod. 1996, 59, 406–408. [CrossRef]
376. Tsukitani, Y.; Kawanishi, S.; Shoji, J. Studies on the constituents of Senegae Radix. II. The structure of senegin-II,
a saponin from Polygala senega latifolia Torry et Gray. Chem. Pharm. Bull. 1973, 21, 791–799. [CrossRef]
377. Tsukitani, Y.; Shoji, J. Studies on the constituents of Senegae Radix. III. The structures of senegin-III and -IV,
saponins from Polygala senega Linne var. latifolia Torry et Gray. Chem. Pharm. Bull. 1973, 21, 1564–1574.
[CrossRef]
378. Saitoh, H.; Miyase, T.; Ueno, A. Senegoses A-E, oligosaccharide multi-esters from Polygala senega var. latifolia
Torr. et Gray. Chem. Pharm. Bull. 1993, 41, 1127–1131. [CrossRef] [PubMed]
379. Saitoh, H.; Miyase, T.; Ueno, A. Senegoses F-I, oligosaccharide multi-esters from the roots of Polygala senega
var. latifolia Torr. et Gray. Chem. Pharm. Bull. 1993, 41, 2125–2128. [CrossRef] [PubMed]
380. Saitoh, H.; Miyase, T.; Ueno, A.; Atarashi, K.; Saiki, Y. Senegoses J-O, oligosaccharide multi-esters from the
roots of Polygala senega L. Chem. Pharm. Bull. 1994, 43, 641–645. [CrossRef]
381. Yoshikawa, M.; Murakami, T.; Ueno, T.; Kadoya, M.; Matsuda, H.; Yamahara, J.; Murakami, N.
E-Senegasaponins A and B, Z-senegasaponins A and B, Z-senegins II and III, new type inhibitors of ethanol
absorption in rats from Senegae Radix, the roots of Polygala senega L. var latifolia Torrey et Gray. Chem. Pharm.
Bull. 1995, 43, 350–352. [CrossRef] [PubMed]
382. Hayashi, S.; Kameoka, H. Volatile compounds of Polygala senega L. var. latifolia Torrey et Gray. Flavour Fragr.
J. 1995, 10, 273–280. [CrossRef]
383. Arai, M.; Hayashi, A.; Sobou, M.; Ishida, S.; Kawachi, T.; Kotoku, N.; Kobayashi, M. Anti-angiogenic effect of
triterpenoidal saponins from Polygala senega. J. Nat. Med. 2011, 65, 149–156. [CrossRef] [PubMed]
384. Kim, H.J.; Woo, E.-R.; Park, H. A novel lignan and flavonoids from Polygonum aviculare. J. Nat. Prod. 1994, 57,
581–586. [CrossRef]
385. Al-Hazimi, H.M.A.; Haque, S.N. A new naphthoquinone from Polygonum aviculare. Nat. Prod. Lett. 2002, 16,
115–118. [CrossRef] [PubMed]
386. Yunuskhodzhaeva, N.A.; Eshbakova, K.A.; Abdullabekova, V.N. Flavonoid composition of the herb
Polygonum aviculare. Chem. Nat. Compd. 2010, 46, 803–804. [CrossRef]
387. Granica, S.; Czerwi´nska, M.E.; Zyzy´nska-Granica, B.; Kiss, A.K. Antioxidant and anti-inflammatory flavonol
glucuronides from Polygonum aviculare L. Fitoterapia 2013, 91, 180–188. [CrossRef] [PubMed]
388. Nugroho, A.; Kim, E.J.; Choi, J.S.; Park, H.-J. Simultaneous quantification and peroxynitrite-scavenging
activities of flavonoids in Polygonum aviculare L. herb. J. Pharm. Biomed. Anal. 2014, 89, 93–98. [CrossRef]
[PubMed]
389. Yang, H.H.; Hwangbo, K.; Zheng, M.S.; Cho, J.H.; Son, J.-K.; Kim, H.Y.; Baek, S.H.; Choi, H.C.; Park, S.Y.;
Kim, J.-R. Quercetin-3-O-β-D-glucuronide isolated from Polygonum aviculare inhibits cellular senescence in
human primary cells. Arch. Pharm. Res. 2014, 37, 1219–1233. [CrossRef] [PubMed]
390. Barnes, C.S.; Loder, J.W. The structure of polygodial: A new sesquiterpene dialdehyde from Polygonum
hydropiper L. Aust. J. Chem. 1962, 15, 322–327. [CrossRef]
391. Fukuyama, Y.; Sato, T.; Asakawa, Y.; Takemoto, T. A potent cytotoxic warburganal and related drimane-type
sesquiterpenoids from Polygonum hydropiper. Phytochemistry 1980, 21, 2895–2898. [CrossRef]
392. Yang, X.; Wang, B.-C.; Zhang, X.; Yang, S.-P.; Li, W.; Tang, Q.; Singh, G.K. Simultaneous determination
of nine flavonoids in Polygonum hydropiper L. samples using nanomagnetic powder three-phase hollow
fibre-based liquid-phase microextraction combined with ultrahigh performance liquid chromatography-mass
spectrometry. J. Pharm. Biomed. Anal. 2011, 54, 311–316. [CrossRef] [PubMed]
393. Fukuyama, Y.; Sato, T.; Miura, I.; Asakawa, Y. Drimane-type sesqui- and norsesquiterpenoids from Polygonum
hydropiper. Phytochemistry 1985, 24, 1521–1524. [CrossRef]
394. Haraguchi, H.; Hashimoto, K.; Yagi, A. Antioxidative substances in leaves of Polygonum hydropiper. J. Agric.
Food Chem. 1992, 40, 1349–1351. [CrossRef]
395. Yagi, A.; Uemura, T.; Okamura, N.; Haraguchi, H.; Imoto, T.; Hashimoto, K. Antioxidative sulphated
flavonoids in leaves of Polygonum hydropiper. Phytochemistry 1994, 35, 885–887. [CrossRef]
396. Peng, Z.F.; Strack, D.; Baumert, A.; Subramaniam, R.; Goh, N.K.; Chia, T.F.; Tan, S.N.; Chia, L.S. Antioxidant
flavonoids from leaves of Polygonum hydropiper L. Phytochemistry 2003, 62, 219–228. [CrossRef]
397. Haraguchi, H.; Matsuda, R.; Hashimoto, K. High-performance liquid chromatographic determination of
sesquiterpene dialdehydes and antifungal activity from Polygonum hydropiper. J. Agric. Food Chem. 1993, 41,
5–7. [CrossRef]
398. Miyazawa, M.; Tamura, N. Inhibitory compound of tyrosinase activity from the sprout of Polygonum
hydropiper L. (Benitade). Biol. Pharm. Bull. 2007, 30, 595–597. [CrossRef] [PubMed]
399. Van Kiem, P.; Nhiem, N.X.; Cuong, N.X.; Hoa, T.Q.; Huong, H.T.; Huong, L.M.; Van Minh, C.; Kim, Y.H. New
phenylpropanoid esters of sucrose from Polygonum hydropiper and their antioxidant activity. Arch. Pharm.
Res. 2008, 31, 1477–1482. [CrossRef] [PubMed]
400. Miyazawa, M.; Tamura, N. Components of the essential oil from sprouts of Polygonum hydropiper L.
(‘Benitade’). Flavour Fragr. J. 2007, 22, 188–190. [CrossRef]
401. Maheswaran, R.; Ignacimuthu, S. Bioefficacy of essential oil from Polygonum hydropiper L. against mosquitoes,
Anopheles stephensi and Culex quinquefasciatus. Ecotoxicol. Environ. Saf. 2013, 97, 26–31. [CrossRef] [PubMed]
402. Morteza-Semnani, K.; Saeedi, M.; Akbarzadeh, M. The essential oil composition of Prunella vulgaris L. J.
Essent. Oil Bear. Plants 2006, 9, 257–260. [CrossRef]
403. Chen, Y.; Guo, Q.; Zhu, Z.; Zhang, L.; Dai, X. Comparative analysis of the essential oil of flowers, leaves and
stems of Prunella vulgaris L. J. Essent. Oil Bear. Plants 2012, 15, 662–666. [CrossRef]
404. Chen, Y.; Zhu, Z.; Guo, Q.; Zhang, L.; Zhang, X. Variation in concentrations of major bioactive compounds in
Prunella vulgaris L. related to plant parts and phenological stages. Biol. Res. 2012, 45, 171–175. [CrossRef]
[PubMed]
405. Chen, Y.; Yu, M.; Zhu, Z.; Zhang, L.; Guo, Q. Optimisation of potassium chloride nutrition for proper growth,
physiological development and bioactive component production in Prunella vulgaris L. PLoS ONE 2013,
8, e66259. [CrossRef] [PubMed]
406. Ryu, S.Y.; Oak, M.-H.; Yoon, S.-K.; Cho, D.-I.; Yoo, G.-S.; Kim, T.-S.; Kim, K.-M. Anti-allergic and
anti-inflammatory triterpenes from the herb of Prunella vulgaris. Planta Med. 2000, 66, 358–360. [CrossRef]
[PubMed]
407. Yoon, M.Y.; Choi, G.J.; Choi, Y.H.; Jang, K.S.; Park, M.S.; Cha, B.; Kim, J.C. Effect of polyacetylenic acids from
Prunella vulgaris on various plant pathogens. Lett. Appl. Microbiol. 2010, 51, 511–517. [CrossRef] [PubMed]
408. Gu, X.-J.; Li, Y.-B.; Li, P.; Qian, S.-H.; Zhang, J.-F. Triterpenoid saponins from the spikes of Prunella vulgaris.
Helv. Chim. Acta 2007, 90, 72–78. [CrossRef]
409. Lee, I.K.; Kim, D.H.; Lee, S.Y.; Kim, K.R.; Choi, S.U.; Hong, J.K.; Lee, J.H.; Park, Y.H.; Lee, K.R. Triterpenoic
acids of Prunella vulgaris var. lilacina and their cytotoxic activities in vitro. Arch. Pharm. Res. 2008, 31,
1578–1583. [PubMed]
410. Wang, Z.J.; Zhao, Y.Y.; Wang, B.; Ai, T.M.; Chen, Y.Y. Depsides from Prunella vulgaris. Chin. Chem. Lett. 2000,
11, 997–1001.
411. ¸Sahin, S.; Demir, C.; Malyer, H. Determination of phenolic compounds in Prunella L. by liquid
chromatography-diode array detection. J. Pharm. Biomed. Anal. 2011, 55, 1227–1230. [CrossRef] [PubMed]
412. Gu, X.; Li, Y.; Mu, J.; Zhang, Y. Chemical constituents of Prunella vulgaris. J. Environ. Sci. 2013, 25, S161–S163.
[CrossRef]
413. Olszewska, M. Flavonoids from Prunus serotina Ehrh. Acta Pol. Pharm. Drug Res. 2005, 62, 127–133.
414. Olszewska, M. Quantitative HPLC analysis of flavonoids and chlorogenic acid in the leaves and
inflorescences of Prunus serotina Ehrh. Acta Chromatogr. 2007, 19, 253–269.
415. Olszewska, M. Optimization and validation of an HPLC-UV method for analysis of corosolic, oleanolic,
and ursolic acids in plant material: Application to Prunus serotina Ehrh. Acta Chromatogr. 2008, 20, 643–659.
[CrossRef]
416. Ibarra-Alvarado, C.; Rojas, A.; Luna, F.; Rojas, J.I.; Rivero-Cruz, B.; Rivero-Cruz, J.F. Vasorelaxant constituents
of the leaves of Prunus serotina “capulín”. Rev. Latinoam. Quim. 2009, 37, 164–173.
417. Rivero-Cruz, B. Simultaneous quantification by HPLC of the phenolic compounds for the crude drug of
Prunus serotina subsp. capuli. Pharm. Biol. 2014, 52, 1015–1020. [CrossRef] [PubMed]
418. Biessels, H.W.A.; van der Kerk-van Hoof, A.C.; Kettenes-van den Bosch, J.J.; Salemink, C.A. Triterpenes of
Prunus serotina and P. lusitanica. Phytochemistry 1974, 13, 203–207. [CrossRef]
419. Omar, S.; Lalonde, M.; Marcotte, M.; Cook, M.; Proulx, J.; Goel, K.; Durst, T.; Philogène, B.J.R.;
Arnason, J.T. Insect growth-reducing and antifeedant activity in eastern North America hardwood species
and bioassay-guided isolation of active principles from Prunus serotina. Agric. For. Entomol. 2000, 2, 253–257.
[CrossRef]
420. Hänsel, R.; Ohlendorf, D.; Pelter, A. Obtusifolin, ein Flavanon mit einem biogenetisch unüblichen
C9-Baustein. Z. Naturforsch. B 1970, 25, 989–994. [CrossRef] [PubMed]
421. Wagner, H.; Maurer, G.; Farkas, L.; Hänsel, R.; Ohlendorf, D. Zur Struktur und Synthese von Gnaphaliin,
Methyl-gnaphaliin aus Gnaphalium obtusifolium L. und Isognaphaliin aus Achrocline satureoides. Chem. Ber.
1971, 104, 1281–1288. [CrossRef]
422. Ohlendorf, D.; Schwarz, R.; Hänsel, R. 3,5,7-Trihydroxy-6,8-dimethoxyflavon aus Gnaphalium obtusifolium.
Arch. Pharm. 1971, 304, 213–215. [CrossRef]
423. Murata, T.; Nakano, M.; Miyase, T.; Yoshizaki, F. Chemical constituents of aerial parts and roots of
Pycnanthemum flexuosum. Chem. Pharm. Bull. 2014, 62, 608–612. [CrossRef] [PubMed]
424. Beebe, C.W.; Luvisi, F.P.; Happich, M.L. Tennessee Valley oak bark as a source of tannin. J. Am. Leather Chem.
Assoc. 1953, 48, 32–41.
425. Bai, Y.; Benn, M.H.; Majak, W.; McDiarmid, R. Extraction and HPLC determination of ranunculin in species
of the buttercup family. J. Agric. Food Chem. 1996, 44, 2235–2238. [CrossRef]
426. Mekala, A.B.; Satyal, P.; Setzer, W.N. Phytochemicals from the bark of Rhamnus caroliniana. Nat. Prod.
Commun. 2017, 12, 403–406.
427. Saxena, G.; McCutcheon, A.R.; Farmer, S.; Towers, G.H.N.; Hancock, R.E.W. Antimicrobial constituents of
Rhus glabra. J. Ethnopharmacol. 1994, 42, 95–99. [CrossRef]
428. Heckman, R.A. The Isolation and Identification of Organic Compounds from Rhus glabra. Ph.D. Thesis,
Georgia Institute of Technology, Atlanta, GA, USA, 1965.
429. Wu, T.; McCallum, J.L.; Wang, S.; Liu, R.; Zhu, H.; Tsao, R. Evaluation of antioxidant activities and chemical
characterisation of staghorn sumac fruit (Rhus hirta L.). Food Chem. 2013, 138, 1333–1340. [CrossRef]
[PubMed]
430. Peng, Y.; Zhang, H.; Liu, R.; Mine, Y.; McCallum, J.; Kirby, C.; Tsao, R. Antioxidant and anti-inflammatory
activities of pyranoanthocyanins and other polyphenols from staghorn sumac (Rhus hirta L.) in Caco-2 cell
models. J. Funct. Foods 2016, 20, 139–147. [CrossRef]
431. Van Damme, E.J.M.; Barre, A.; Smeets, K.; Torrekens, S.; Van Leuven, F.; Rougé, P.; Peumans, W.J. The bark of
Robinia pseudoacacia contains a complex mixture of lectins. Characterization of the proteins and the cDNA
clones. Plant Physiol. 1995, 107, 833–843. [CrossRef] [PubMed]
432. Rabijns, A.; Verboven, C.; Rougé, P.; Barre, A.; Van Damme, E.J.M.; Peumans, W.J.; De Ranter, C.J. Structure
of a legume lectin from the bark of Robinia pseudoacacia and its complex with N-acetylgalactosamine. Proteins
Struct. Funct. Genet. 2001, 44, 470–478. [CrossRef] [PubMed]
433. Tian, F.; McLaughlin, J.L. Bioactive flavonoids from the black locust tree, Robinia pseudoacacia. Pharm. Biol.
2000, 38, 229–234. [CrossRef]
434. Veitch, N.C.; Elliott, P.C.; Kite, G.C.; Lewis, G.P. Flavonoid glycosides of the black locust tree, Robinia
pseudoacacia (Leguminosae). Phytochemistry 2010, 71, 479–486. [CrossRef] [PubMed]
435. Duverger, E.; Delmotte, F.M. Purification of lectins from Robinia pseudoacacia L. root-tips. Plant Sci. 1997, 123,
9–18. [CrossRef]
436. Ono, M.; Yasuda, S.; Komatsu, H.; Fujiwara, Y.; Takeya, M.; Nohara, T. Triterpenoids from the fruits and
leaves of the blackberry (Rubus allegheniensis) and their inhibitory activities on foam cell formation in human
monocyte-derived macrophage. Nat. Prod. Res. 2014, 28, 2347–2350. [CrossRef] [PubMed]
437. Dvaranauskaite, A.; Venskutonis, P.R.; Labokas, J. Comparison of quercetin derivatives in ethanolic extracts
of red raspberry (Rubus idaeus L.) leaves. Acta Aliment. 2008, 37, 449–461. [CrossRef]
438. Vera, J.R.; Dacke, C.G.; Blunden, G.; Patel, A.V. Smooth muscle relaxant triterpenoid glycosides from Rubus
idaeus (raspberry) leaves. Nat. Prod. Commun. 2006, 1, 705–710.
439. Ferlemi, A.-V.; Lamari, F.N. Berry leaves: An alternative source of bioactive natural products of nutritional
and medicinal value. Antioxidants 2016, 5, 17. [CrossRef] [PubMed]
440. Stewart, C.D.; Jones, C.D.; Setzer, W.N. Leaf essential oil compositions of Rudbeckia fulgida Aiton, Rudbeckia
hirta L., and Symphyotrichum novae-angliae (L.) G.L. Nesom (Asteraceae). Am. J. Essent. Oils Nat. Prod. 2014, 2,
36–38.
441. Lee, S.Y.; Woo, K.W.; Kim, C.S.; Lee, D.U.; Lee, K.R. New lignans from the aerial parts of Rudbeckia laciniata.
Helv. Chim. Acta 2013, 96, 320–325. [CrossRef]
442. Lee, S.Y.; Shin, Y.J.; Choi, S.U.; Lee, K.R. A new flavonol glycoside from the aerial part of Rudbeckia laciniata.
Arch. Pharm. Res. 2014, 37, 834–838. [CrossRef] [PubMed]
443. Bohlmann, F.; Jakupovic, J.; Zdero, C. Neue Norsesquiterpene aus Rudbeckia laciniata und Senecio paludaffinis.
Phytochemistry 1978, 17, 2034–2036. [CrossRef]
444. Jakupovic, J.; Jia, Y.; King, R.M.; Bohlmann, F. Rudbeckiolid, ein dimeres Sesquiterpenlacton aus Rudbeckia
laciniata. Justus Liebigs Ann. Chem. 1986, 8, 1474–1477. [CrossRef]
445. Fukushi, Y.; Yajima, C.; Mizutani, J.; Tahara, S. Tricyclic sesquiterpenes from Rudbeckia laciniata. Phytochemistry
1998, 49, 593–600. [CrossRef]
446. Sando, C.E.; Lloyd, J.U. The isolation and identification of rutin from the flowers of elder
(Sambucus canadensis L.). J. Biol. Chem. 1924, 58, 737–745.
447. Inami, O.; Tamura, I.; Kikuzaki, H.; Nakatani, N. Stability of anthocyanins of Sambucus canadensis and
Sambucus nigra. J. Agric. Food Chem. 1996, 44, 3090–3096. [CrossRef]
448. Lee, J.; Finn, C.E. Anthocyanins and other polyphenolics in American elderberry (Sambucus canadensis) and
European elderberry (S. nigra) cultivars. J. Sci. Food Agric. 2007, 87, 2665–2675. [CrossRef] [PubMed]
449. Nakatani, N.; Kikuzaki, H.; Hikida, J.; Ohba, M.; Inami, O.; Tamura, I. Acylated anthocyanins from fruits of
Sambucus canadensis. Phytochemistry 2013, 38, 755–757. [CrossRef]
450. Greathouse, G.A. Alkaloids from Sanguinaria canadensis and their influence on growth of Phymatotrichum
omnivorum. Plant Physiol. 1939, 14, 377–380. [CrossRef] [PubMed]
451. Salmore, A.K.; Hunter, M.D. Environmental and genotypic influences on isoquinoline alkaloid content in
Sanguinaria canadensis. J. Chem. Ecol. 2001, 27, 1729–1747. [CrossRef] [PubMed]
452. Newton, S.M.; Lau, C.; Gurcha, S.S.; Besra, G.S.; Wright, C.W. The evaluation of forty-three plant species for
in vitro antimycobacterial activities; isolation of active constituents from Psoralea corylifolia and Sanguinaria
canadensis. J. Ethnopharmacol. 2002, 79, 57–67. [CrossRef]
453. Mahady, G.B.; Pendland, S.L.; Stoia, A.; Chadwick, L.R. In vitro susceptibility of Helicobacter pylori to
isoquinoline alkaloids from Sanguinaria canadensis and Hydrastis canadensis. Phyther. Res. 2003, 17, 217–221.
[CrossRef] [PubMed]
454. Graf, T.N.; Levine, K.E.; Andrews, M.E.; Perlmutter, J.M.; Nielsen, S.J.; Davis, J.M.; Wani, M.C.; Oberlies, N.H.
Variability in the yield of benzophenanthridine alkaloids in wildcrafted vs. cultivated bloodroot (Sanguinaria
canadensis L.). J. Agric. Food Chem. 2007, 55, 1205–1211. [CrossRef] [PubMed]
455. Croaker, A.; King, G.J.; Pyne, J.H.; Anoopkumar-Dukie, S.; Liu, L. Sanguinaria canadensis: Traditional
medicine, phytochemical composition, biological activities and current uses. Int. J. Mol. Sci. 2016, 17, 1414.
[CrossRef] [PubMed]
456. Kaler, K.M.; Setzer, W.N. Seasonal variation in the leaf essential oil composition of Sassafras albidum. Nat.
Prod. Commun. 2008, 3, 829–832.
457. Kamdem, D.P.; Gage, D.A. Chemical composition of essential oil from the root bark of Sassafras albidum.
Planta Med. 1995, 61, 574–575. [CrossRef] [PubMed]
458. Kennedy, J.E.; Davé, P.C.; Harbin, L.N.; Setzer, W.N. Allelopathic potential of Sassafras albidum and Pinus
taeda essential oils. Allelopath. J. 2011, 27, 111–122.
459. Pulivarthi, D.; Steinberg, K.M.; Monzote, L.; Piñón, A.; Setzer, W.N. Antileishmanial activity of compounds
isolated from Sassafras albidum. Nat. Prod. Commun. 2015, 10, 1229–1230. [PubMed]
460. Rao, K.V.; Alvarez, F.M. Chemistry of Saururus cernuus. I. Saucernetin, a new neolignan. J. Nat. Prod. 1982,
45, 393–397. [CrossRef]
461. Rao, K.V.; Alvarez, F.M. Manassantins A/B and saucerneol: Novel biologically active lignoids from Saururus
cernuus. Tetrahedron Lett. 1983, 24, 4947–4950. [CrossRef]
462. Rao, K.V.; Reddy, G.C.S. Chemistry of Saururus cernuus, V. Sauristolactam and other nitrogenous constituents.
J. Nat. Prod. 1990, 53, 309–312. [CrossRef] [PubMed]
463. Rao, K.V.; Prakasa Rao, N.S. Chemistry of Saururus cernuus, VI: Three new neolignans. J. Nat. Prod. 1990, 53,
212–215. [CrossRef] [PubMed]
464. Kubanek, J.; Fenical, W.; Hay, M.E.; Brown, P.J.; Lindquist, N. Two antifeedant lignans from the freshwater
macrophyte Saururus cernuus. Phytochemistry 2000, 54, 281–287. [CrossRef]
465. Kubanek, J.; Hay, M.E.; Brown, P.J.; Lindquist, N.; Fenical, W. Lignoid chemical defenses in the freshwater
macrophyte Saururus cernuus. Chemoecology 2001, 11, 1–8. [CrossRef]
466. Rajbhandari, I.; Takamatsu, S.; Nagle, D.G. A new dehydrogeranylgeraniol antioxidant from Saururus cernuus
that inhibits intracellular reactive oxygen species (ROS)-catalyzed oxidation within HL-60 cells. J. Nat. Prod.
2001, 64, 693–695. [CrossRef] [PubMed]
467. Hodges, T.W.; Hossain, C.F.; Kim, Y.-P.; Zhou, Y.-D.; Nagle, D.G. Molecular-targeted antitumor agents: The
Saururus cernuus dineolignans manassantin B and 4-O-demethylmanassantin B are potent inhibitors of
hypoxia-activated HIF-1. J. Nat. Prod. 2004, 67, 767–771. [CrossRef] [PubMed]
468. Hossain, C.F.; Kim, Y.-P.; Baerson, S.R.; Zhang, L.; Bruick, R.K.; Mohammed, K.A.; Agarwal, A.K.; Nagle, D.G.;
Zhou, Y.D. Saururus cernuus lignans—Potent small molecule inhibitors of hypoxia-inducible factor-1. Biochem.
Biophys. Res. Commun. 2005, 333, 1026–1033. [CrossRef] [PubMed]
469. Upton, R.; DAyu, R.H. Skullcap Scutellaria lateriflora L.: An American nervine. J. Herb. Med. 2012, 2, 76–96.
[CrossRef]
470. Yaghmai, M.S. Volatile constituents of Scutellaria lateriflora L. Flavour Fragr. J. 1988, 3, 27–31. [CrossRef]
471. Bruno, M.; Cruciata, M.; Bondi, M.L.; Piozzi, F.; de la Torre, M.; Rodgriguez, B.; Servettaz, O. Neo-clerodane
diterpenoids from Scutellaria lateriflora. Phytochemistry 1998, 48, 687–691. [CrossRef]
472. Awad, R.; Arnason, J.T.; Trudeau, V.; Bergeron, C.; Budzinski, J.W.; Foster, B.C.; Merali, Z. Phytochemical
and biological analysis of skullcap (Scutellaria lateriflora L.): A medicinal plant with anxiolytic properties.
Phytomedicine 2003, 10, 640–649. [CrossRef] [PubMed]
473. Cole, I.B.; Cao, J.; Alan, A.R.; Saxena, P.K.; Murch, S.J. Comparisons of Scutellaria baicalensis, Scutellaria
lateriflora and Scutellaria racemosa: Genome size, antioxidant potential and phytochemistry. Planta Med. 2008,
74, 474–481. [CrossRef] [PubMed]
474. Zhang, Z.; Lian, X.Y.; Li, S.; Stringer, J.L. Characterization of chemical ingredients and anticonvulsant activity
of American skullcap (Scutellaria lateriflora). Phytomedicine 2009, 16, 485–493. [CrossRef] [PubMed]
475. Li, J.; Ding, Y.; Li, X.; Ferreira, D.; Khan, S.; Smillie, T.; Khan, I.A. Scuteflorins A and B,
dihydropyranocoumarins from Scutellaria lateriflora. J. Nat. Prod. 2009, 72, 983–987. [CrossRef] [PubMed]
476. Islam, M.N.; Downey, F.; Ng, C.K.Y. Comparative analysis of bioactive phytochemicals from Scutellaria
baicalensis, Scutellaria lateriflora, Scutellaria racemosa, Scutellaria tomentosa and Scutellaria wrightii by
LC-DAD-MS. Metabolomics 2011, 7, 446–453. [CrossRef]
477. Kuroda, M.; Iwabuchi, K.; Mimaki, Y. Chemical constituents of the aerial parts of Scutellaria lateriflora and
their α-glucosidase inhibitory activities. Nat. Prod. Commun. 2012, 7, 471–474. [PubMed]
478. Li, J.; Wang, Y.H.; Smillie, T.J.; Khan, I.A. Identification of phenolic compounds from Scutellaria lateriflora
by liquid chromatography with ultraviolet photodiode array and electrospray ionization tandem mass
spectrometry. J. Pharm. Biomed. Anal. 2012, 63, 120–127. [CrossRef] [PubMed]
479. Zalkow, L.H.; Gelbaum, L.T.; Van Derveer, D. Eremophilane sesquiterpenes from Senecio aureus. J. Chem. Soc.
Perkin Trans. 1979, 1542–1546. [CrossRef]
480. Williams, J.D. The Flavonoids and Phenolic Acids of the Genus Silphium and Their Chemosystematic and
Medicinal Value. Ph.D. Thesis, University of Texas, Austin, TX, USA, 2006.
481. Thacker, J.D.; Bordner, J.; Bumgardner, C. Carolinoside: A phytosteroidal glycoside from Solanum carolinense.
Phytochemistry 1990, 29, 2965–2970. [CrossRef]
482. Evans, W.C.; Somanabandhu, A. Bases from roots of Solanum carolinense. Phytochemistry 1977, 16, 1859–1860.
[CrossRef]
483. Tucker, A.O.; Maciarello, M.J.; Clancy, K. Sweet goldenrod (Solidago odora, Asteraceae): A medicine, tea, and
state herb. Econ. Bot. 1999, 53, 281–284. [CrossRef]
484. Adolf, W.; Hecker, E. New irritant diterpene-esters from roots of Stillingia sylvatica L. (Euphorbiaceae).
Tetrahedron Lett. 1980, 21, 2887–2890. [CrossRef]
485. Shamma, M.; Rothenberg, A.S. The alkaloids of Thalictrum dioicum. Lloydia 1978, 41, 169–178.
486. Shamma, M.; Rothenberg, A.S.; Salgar, S.S.; Jayatilake, G.S. Thalidine, a new isopavine alkaloid from
Thalictrum dioicum. Lloydia 1976, 39, 395–398. [PubMed]
487. Shamma, M.; Salgar, S.S. Pallidine and corydine from Thalictrum dioicum. Phytochemistry 1973, 12, 1505–1506.
[CrossRef]
488. Pérez-Ortega, G.; Guevara-Fefer, P.; Chávez, M.; Herrera, J.; Martínez, A.; Martínez, A.L.;
González-Trujano, M.E. Sedative and anxiolytic efficacy of Tilia americana var. mexicana inflorescences
used traditionally by communities of State of Michoacan, Mexico. J. Ethnopharmacol. 2008, 116, 461–468.
489. Herrera-Ruiz, M.; Román-Ramos, R.; Zamilpa, A.; Tortoriello, J.; Jiménez-Ferrer, J.E. Flavonoids from Tilia
americana with anxiolytic activity in plus-maze test. J. Ethnopharmacol. 2008, 118, 312–317. [CrossRef]
[PubMed]
490. Martínez, A.L.; González-Trujano, M.E.; Aguirre-Hernández, E.; Moreno, J.; Soto-Hernández, M.;
López-Muñoz, F.J. Antinociceptive activity of Tilia americana var. mexicana inflorescences and quercetin in
the formalin test and in an arthritic pain model in rats. Neuropharmacology 2009, 56, 564–571.
491. Aguirre-Hernández, E.; González-Trujano, M.E.; Martínez, A.L.; Moreno, J.; Kite, G.; Terrazas, T.;
Soto-Hernández, M. HPLC/MS analysis and anxiolytic-like effect of quercetin and kaempferol flavonoids
from Tilia americana var. mexicana. J. Ethnopharmacol. 2010, 127, 91–97.
492. Cardenas-Rodriguez, N.; Gonzalez-Trujano, M.E.; Aguirre-Hernandez, E.; Ruiz-Garcia, M.; Sampieri, A.;
Coballase-Urrutia, E.; Carmona-Aparicio, L. Anticonvulsant and antioxidant effects of Tilia americana var.
mexicana and flavonoids constituents in the pentylenetetrazole-induced seizures. Oxid. Med. Cell. Longev.
2014, 2014. [CrossRef] [PubMed]
493. Shaw, A.C. The essential oil of Tsuga canadensis (L.) Carr. J. Am. Chem. Soc. 1951, 73, 2859–2861. [CrossRef]
494. Lagalante, A.F.; Montgomery, M.E. Analysis of terpenoids from hemlock (Tsuga) species by solid-phase
microextraction/gas chromatography/ion-trap mass spectrometry. J. Agric. Food Chem. 2003, 51, 2115–2120.
[CrossRef] [PubMed]
495. Lagalante, A.F.; Lewis, N.; Montgomery, M.E.; Shields, K.S. Temporal and spatial variation of terpenoids in
eastern hemlock (Tsuga canadensis) in relation to feeding by Adelges tsugae. J. Chem. Ecol. 2006, 32, 2389–2403.
[CrossRef] [PubMed]
496. Lagalante, A.F.; Montgomery, M.E.; Calvosa, F.C.; Mirzabeigi, M.N. Characterization of terpenoid volatiles
from cultivars of eastern hemlock (Tsuga canadensis). J. Agric. Food Chem. 2007, 55, 10850–10856. [CrossRef]
[PubMed]
497. Craft, J.D.; Setzer, W.N. Leaf essential oil composition of Tsuga canadensis growing wild in North Alabama
and Northwest Georgia. Am. J. Essent. Oils Nat. Prod. 2017, 5, 26–29.
498. Horhammer, L.; Wagner, H.; Reinhardt, H. Isoleuring des Bis-(5,7,4 -trihydroxy-)-flavons, Amentoflavon
aus der Rinde von Viburnum prunifolium L. (Amerikan Schneeball). Naturwissenschaften 1965, 7, 161–162.
[CrossRef]
499. Cometa, M.F.; Parisi, L.; Palmery, M.; Meneguz, A.; Tomassini, L. In vitro relaxant and spasmolytic effects
of constituents from Viburnum prunifolium and HPLC quantification of the bioactive isolated iridoids. J.
Ethnopharmacol. 2009, 123, 201–207. [CrossRef] [PubMed]
500. Jarboe, C.H.; Zirvi, K.A.; Schmidt, C.M.; McLafferty, F.W.; Haddon, W.F. 1-Methyl 2,3-dibutyl hemimellitate,
a novel component of Viburnum prunifolium. J. Org. Chem. 1969, 34, 4202–4203. [CrossRef] [PubMed]
501. Lopez, E.M.; Craft, J.D.; Setzer, W.N. Volatile composition of Vicia caroliniana growing in Huntsville, Alabama.
Am. J. Essent. Oils Nat. Prod. 2017, 5, 8–10.
502. Hussein, F.T. An Investigation of the Alkaloids of Xanthorhiza simplicissima Marsh. Ph.D. Thesis, The Ohio
State University, Columbus, OH, USA, 1963.
503. Okunade, A.L.; Hufford, C.D.; Richardson, M.D.; Peterson, J.R.; Clark, A.M. Antimicrobial properties of
alkaloids from Xanthorhiza simplicissima. J. Pharm. Sci. 1994, 83, 404–406. [CrossRef] [PubMed]
504. Knapp, J.E.; Hussein, F.T.; Beal, J.L.; Doskotch, R.W.; Tomimatsu, T. Isolation of two bisbenzylisoquinoline
alkaloids from the rhizomes and roots of Xanthorhiza simplicissima. J. Pharm. Sci. 1967, 56, 139–141. [CrossRef]
[PubMed]
505. Ju, Y.; Still, C.C.; Sacalis, J.N.; Li, J.; Ho, C.T. Cytotoxic coumarins and lignans from extracts of the northern
prickly ash (Zanthoxylum americanum). Phyther. Res. 2001, 15, 441–443. [CrossRef] [PubMed]
506. Eiter, L.C.; Fadamiro, H.; Setzer, W.N. Seasonal variation in the leaf essential oil composition of Zanthoxylum
clava-herculis growing in Huntsville, Alabama. Nat. Prod. Commun. 2010, 5, 457–460. [PubMed]
507. Steinberg, K.M.; Satyal, P.; Setzer, W.N. Bark essential oils of Zanthoxylum clava-herculis and Ptelea trifoliata:
Enantiomeric distribution of monoterpenoids. Nat. Prod. Commun. 2017, 12, 961–963.
508. Rao, K.V.; Davies, R. The ichthyotoxic principles of Zanthoxylum clava-herculis. J. Nat. Prod. 1986, 49, 340–342.
[CrossRef]
509. Gibbons, S.; Leimkugel, J.; Oluwatuyi, M.; Heinrich, M. Activity of Zanthoxylum clava-herculis extracts against
multi-drug resistant methicillin-resistant Staphylococcus aureus (mdr-MRSA). Phyther. Res. 2003, 17, 274–275.
[CrossRef] [PubMed]
510. Chandler, R.F.; Hooper, S.N.; Harvey, M.J. Ethnobotany and phytochemistry of yarrow, Achillea millefolium,
Compositae. Econ. Bot. 1982, 36, 203–223. [CrossRef]
511. Bruneton, J. Pharmacognosy, 2nd ed.; Intercept Ltd.: London, UK, 1999.
512. Ali, S.I.; Gopalakrishnan, B.; Venkatesalu, V. Pharmacognosy, phytochemistry and pharmacological
properties of Achillea millefolium L.: A review. Phyther. Res. 2017, 31, 1140–1161. [CrossRef] [PubMed]
513. Borrelli, F.; Romano, B.; Fasolino, I.; Tagliatatela-Scafati, O.; Aprea, G.; Capasso, R.; Capasso, F.; Coppola
Bottazzi, E.; Izzo, A.A. Prokinetic effect of a standardized yarrow (Achillea millefolium) extract and its
constituent choline: Studies in the mouse and human stomach. Neurogastroenterol. Motil. 2012, 24, 164–172.
[CrossRef] [PubMed]
514. Hajhashemi, M.; Ghanbari, Z.; Movahedi, M.; Rafieian, M.; Keivani, A.; Haghollahi, F. The effect of Achillea
millefolium and Hypericum perforatum ointments on episiotomy wound healing in primiparous women. J.
Matern. Neonatal Med. 2018, 31, 63–69. [CrossRef] [PubMed]
515. Chen, W.-C.; Liou, S.-S.; Tzeng, T.-F.; Lee, S.-L.; Liu, I.-M. Effect of topical application of chlorogenic acid on
excision wound healing in rats. Planta Med. 2013, 79, 616–621. [CrossRef] [PubMed]
516. Bagdas, D.; Etoz, B.C.; Gul, Z.; Ziyanok, S.; Inan, S.; Turacozen, O.; Gul, N.Y.; Topal, A.; Cinkilic, N.;
Tas, S.; et al. In vivo systemic chlorogenic acid therapy under diabetic conditions: Wound healing effects and
cytotoxicity/genotoxicity profile. Food Chem. Toxicol. 2015, 81, 54–61. [CrossRef] [PubMed]
517. Süntar, I.; Akkol, E.K.; Keles, H.; Yesilada, E.; Sarker, S.D. Exploration of the wound healing potential of
Helichrysum graveolens (Bieb.) Sweet: Isolation of apigenin as an active component. J. Ethnopharmacol. 2013,
149, 103–110. [CrossRef] [PubMed]
518. Lopez-Jornet, P.; Camacho-Alonso, F.; Gómez-Garcia, F.; Molina Miñano, F.; Cañas, X.; Serafín, A.; Castillo, J.;
Vicente-Ortega, V. Effects of potassium apigenin and Verbena extract on the wound healing process of SKH-1
mouse skin. Int. Wound J. 2014, 11, 489–495. [CrossRef] [PubMed]
519. Manivannan, R. Isolation of apigenin-7-O-(6”-O-E-caffeoyl)-β-D-glucopyranoside from Leucas aspera L. with
anti-inflammatory and wound healing activities. J. Pharm. Pharmacogn. Res. 2016, 4, 54–61.
520. Lodhi, S.; Singhai, A.K. Wound healing effect of flavonoid rich fraction and luteolin isolated from Martynia
annua Linn. on streptozotocin induced diabetic rats. Asian Pac. J. Trop. Med. 2013, 6, 253–259. [CrossRef]
499. Cometa, M.F.; Parisi, L.; Palmery, M.; Meneguz, A.; Tomassini, L. In vitro relaxant and spasmolytic effects
of constituents from Viburnum prunifolium and HPLC quantification of the bioactive isolated iridoids. J.
Ethnopharmacol. 2009, 123, 201–207. [CrossRef] [PubMed]
500. Jarboe, C.H.; Zirvi, K.A.; Schmidt, C.M.; McLafferty, F.W.; Haddon, W.F. 1-Methyl 2,3-dibutyl hemimellitate,
a novel component of Viburnum prunifolium. J. Org. Chem. 1969, 34, 4202–4203. [CrossRef] [PubMed]
501. Lopez, E.M.; Craft, J.D.; Setzer, W.N. Volatile composition of Vicia caroliniana growing in Huntsville, Alabama.
Am. J. Essent. Oils Nat. Prod. 2017, 5, 8–10.
502. Hussein, F.T. An Investigation of the Alkaloids of Xanthorhiza simplicissima Marsh. Ph.D. Thesis, The Ohio
State University, Columbus, OH, USA, 1963.
503. Okunade, A.L.; Hufford, C.D.; Richardson, M.D.; Peterson, J.R.; Clark, A.M. Antimicrobial properties of
alkaloids from Xanthorhiza simplicissima. J. Pharm. Sci. 1994, 83, 404–406. [CrossRef] [PubMed]
504. Knapp, J.E.; Hussein, F.T.; Beal, J.L.; Doskotch, R.W.; Tomimatsu, T. Isolation of two bisbenzylisoquinoline
alkaloids from the rhizomes and roots of Xanthorhiza simplicissima. J. Pharm. Sci. 1967, 56, 139–141. [CrossRef]
[PubMed]
505. Ju, Y.; Still, C.C.; Sacalis, J.N.; Li, J.; Ho, C.T. Cytotoxic coumarins and lignans from extracts of the northern
prickly ash (Zanthoxylum americanum). Phyther. Res. 2001, 15, 441–443. [CrossRef] [PubMed]
506. Eiter, L.C.; Fadamiro, H.; Setzer, W.N. Seasonal variation in the leaf essential oil composition of Zanthoxylum
clava-herculis growing in Huntsville, Alabama. Nat. Prod. Commun. 2010, 5, 457–460. [PubMed]
507. Steinberg, K.M.; Satyal, P.; Setzer, W.N. Bark essential oils of Zanthoxylum clava-herculis and Ptelea trifoliata:
Enantiomeric distribution of monoterpenoids. Nat. Prod. Commun. 2017, 12, 961–963.
508. Rao, K.V.; Davies, R. The ichthyotoxic principles of Zanthoxylum clava-herculis. J. Nat. Prod. 1986, 49, 340–342.
[CrossRef]
509. Gibbons, S.; Leimkugel, J.; Oluwatuyi, M.; Heinrich, M. Activity of Zanthoxylum clava-herculis extracts against
multi-drug resistant methicillin-resistant Staphylococcus aureus (mdr-MRSA). Phyther. Res. 2003, 17, 274–275.
[CrossRef] [PubMed]
510. Chandler, R.F.; Hooper, S.N.; Harvey, M.J. Ethnobotany and phytochemistry of yarrow, Achillea millefolium,
Compositae. Econ. Bot. 1982, 36, 203–223. [CrossRef]
511. Bruneton, J. Pharmacognosy, 2nd ed.; Intercept Ltd.: London, UK, 1999.
512. Ali, S.I.; Gopalakrishnan, B.; Venkatesalu, V. Pharmacognosy, phytochemistry and pharmacological
properties of Achillea millefolium L.: A review. Phyther. Res. 2017, 31, 1140–1161. [CrossRef] [PubMed]
513. Borrelli, F.; Romano, B.; Fasolino, I.; Tagliatatela-Scafati, O.; Aprea, G.; Capasso, R.; Capasso, F.; Coppola
Bottazzi, E.; Izzo, A.A. Prokinetic effect of a standardized yarrow (Achillea millefolium) extract and its
constituent choline: Studies in the mouse and human stomach. Neurogastroenterol. Motil. 2012, 24, 164–172.
[CrossRef] [PubMed]
514. Hajhashemi, M.; Ghanbari, Z.; Movahedi, M.; Rafieian, M.; Keivani, A.; Haghollahi, F. The effect of Achillea
millefolium and Hypericum perforatum ointments on episiotomy wound healing in primiparous women. J.
Matern. Neonatal Med. 2018, 31, 63–69. [CrossRef] [PubMed]
515. Chen, W.-C.; Liou, S.-S.; Tzeng, T.-F.; Lee, S.-L.; Liu, I.-M. Effect of topical application of chlorogenic acid on
excision wound healing in rats. Planta Med. 2013, 79, 616–621. [CrossRef] [PubMed]
516. Bagdas, D.; Etoz, B.C.; Gul, Z.; Ziyanok, S.; Inan, S.; Turacozen, O.; Gul, N.Y.; Topal, A.; Cinkilic, N.;
Tas, S.; et al. In vivo systemic chlorogenic acid therapy under diabetic conditions: Wound healing effects and
cytotoxicity/genotoxicity profile. Food Chem. Toxicol. 2015, 81, 54–61. [CrossRef] [PubMed]
517. Süntar, I.; Akkol, E.K.; Keles, H.; Yesilada, E.; Sarker, S.D. Exploration of the wound healing potential of
Helichrysum graveolens (Bieb.) Sweet: Isolation of apigenin as an active component. J. Ethnopharmacol. 2013,
149, 103–110. [CrossRef] [PubMed]
518. Lopez-Jornet, P.; Camacho-Alonso, F.; Gómez-Garcia, F.; Molina Miñano, F.; Cañas, X.; Serafín, A.; Castillo, J.;
Vicente-Ortega, V. Effects of potassium apigenin and Verbena extract on the wound healing process of SKH-1
mouse skin. Int. Wound J. 2014, 11, 489–495. [CrossRef] [PubMed]
519. Manivannan, R. Isolation of apigenin-7-O-(6”-O-E-caffeoyl)-β-D-glucopyranoside from Leucas aspera L. with
anti-inflammatory and wound healing activities. J. Pharm. Pharmacogn. Res. 2016, 4, 54–61.
520. Lodhi, S.; Singhai, A.K. Wound healing effect of flavonoid rich fraction and luteolin isolated from Martynia
annua Linn. on streptozotocin induced diabetic rats. Asian Pac. J. Trop. Med. 2013, 6, 253–259. [CrossRef]
521. Bayrami, Z.; Khalighi-Sigaroodi, F.; Rahimi, R.; Farzaei, M.H.; Hodjat, M.; Baeeri, M.; Rahimifard, M.;
Navaei-Nigjeh, M.; Abdollahi, M.; Hajiaghaee, R. In vitro wound healing activity of luteolin. Res. J.
Pharmacogn. 2017, 4, 7.
522. Ozay, Y.; Guzel, S.; Erdogdu, I.H.; Yildirim, Z.; Pehlivanoglu, B.; Turk, B.A.; Darcan, S. Evaluation of the
wound healing properties of luteolin ointments on excision and incision wound models in diabetic and
non-diabetic rats. Rec. Nat. Prod. 2018, 12, 350–366. [CrossRef]
523. Süntar, I.; Akkol, E.K.; Keles, H.; Yesilada, E.; Sarker, S.D.; Arroo, R.; Baykal, T. Efficacy of Daphne oleoides
subsp. kurdica used for wound healing: Identification of active compounds through bioassay guided isolation
technique. J. Ethnopharmacol. 2012, 141, 1058–1070.
524. Gopalakrishnan, A.; Ram, M.; Kumawat, S.; Tandan, S.; Kumar, D. Quercetin accelerated cutaneous wound
healing in rats by increasing levels of VEGF and TGF-β1. Indian J. Exp. Biol. 2016, 54, 187–195. [PubMed]
525. Ahmad, M.; Sultana, M.; Raina, R.; Pankaj, N.K.; Verma, P.K.; Prawez, S. Hypoglycemic, hypolipidemic, and
wound healing potential of quercetin in streptozotocin-induced diabetic rats. Pharmacogn. Mag. 2017, 13,
S633–S639. [PubMed]
526. Doersch, K.M.; Newll-Rogers, M.K. The impact of quercetin on wound healing relates to changes in αV and
β1 integrin expression. Exp. Biol. Med. 2017, 242, 1424–1431. [CrossRef] [PubMed]
527. Süntar, I.P.; Akkol, E.K.; Yalçin, F.N.; Koca, U.; Kele¸s, H.; Yesilada, E. Wound healing potential of Sambucus
ebulus L. leaves and isolation of an active component, quercetin 3-O-glucoside. J. Ethnopharmacol. 2010, 129,
106–114. [CrossRef] [PubMed]
528. Clericuzio, M.; Tinello, S.; Burlando, B.; Ranzato, E.; Martinotti, S.; Cornara, L.; La Rocca, A. Flavonoid
oligoglycosides from Ophioglossum vulgatum L. Having wound healing properties. Planta Med. 2012, 78,
1639–1644. [CrossRef] [PubMed]
529. Rajamanickam, M.; Kalaivanan, P.; Sivagnanam, I. Antibacterial and wound healing activities of
quercetin-3-O-α-L-rhamnopyranosyl-(1–>6)-β-D-glucopyranoside isolated from Salvia leucantha. Int. J.
Pharm. Sci. Res. 2013, 22, 264–268.
530. Manivannan, R.; Prabakaran, K.; Ilayaraja, S. Isolation, identification and antibacterial and wound healing
studies of quercetin-3-O-α-L-rhamnopyranoside-2”-gallate. Int. J. Appl. Sci. Eng. 2014, 12, 99–106.
531. Seo, S.H.; Lee, S.-H.; Cha, P.-H.; Kim, M.-Y.; Min, D.S.; Choi, K.-Y. Polygonum aviculare L. and its active
compounds, quercitrin hydrate, caffeic acid, and rutin, activate the Wnt/β-catenin pathway and induce
cutaneous wound healing. Phytotherapy 2016, 30, 848–854. [CrossRef] [PubMed]
532. Scott, C.C.; Chen, K.K. The pharmacological action of N-methylcytisine. J. Pharmacol. Exp. Ther. 1943, 79,
334–339.
533. Anonymous. Lupin Alkaloids in Food: A Toxicological Review and Risk Assessment; Australia New Zealand Food
Authority: Canberra, Australia, 2001.
534. Keeler, R.F. Lupin alkaloids from teratogenic and nonteratogenic lupins. III. Identification of anagyrine as
the probable teratogen by feeding trials. J. Toxicol. Environ. Health 1976, 1, 887–898. [CrossRef] [PubMed]
535. de la Peña, J.B.I.; Lee, H.L.; Yoon, S.Y.; Kim, G.H.; Lee, Y.S.; Cheong, J.H. The involvement of magnoflorine
in the sedative and anxiolytic effects of Sinomeni Caulis et Rhizoma in mice. J. Nat. Med. 2013, 67, 814–821.
[CrossRef] [PubMed]
536. Predny, M.L.; De Angelis, P.; Chamberlain, J.L. Black Cohosh, Actaea Racemosa: An Annotated Bibliography; U.S.
Department of Agriculture Forest Service, Southern Research Station: Asheville, NC, USA, 2006.
537. Gruenwald, J.; Brendler, T.; Jaenicke, C. PDR for Herbal Medicines, 4th ed.; Thompson Healthcare, Inc.:
Montvale, NJ, USA, 2007.
538. Liu, Z.; Yang, Z.; Zhu, M.; Huo, J. [Estrogenicity of black cohosh (Cimicifuga racemosa) and its effect on
estrogen receptor level in human breast cancer MCF-7 cells]. Wei Sheng Yan Jiu 2001, 30, 77–80. [PubMed]
539. Seidlová-Wuttke, D.; Hesse, O.; Jarry, H.; Christoffel, V.; Spengler, B.; Becker, T.; Wuttke, W. Evidence for
selective estrogen receptor modulator activity in a black cohosh (Cimicifuga racemosa) extract: Comparison
with estradiol-17β. Eur. J. Endocrinol. 2003, 149, 351–362. [CrossRef] [PubMed]
540. Lupu, R.; Mehmi, I.; Atlas, E.; Tsai, M.-S.; Pisha, E.; Oketch-Rabah, H.A.; Nuntanakorn, P.; Kennelly, E.J.;
Kronenberg, F. Black cohosh, a menopausal remedy, does not have estrogenic activity and does not promote
breast cancer cell growth. Int. J. Oncol. 2003, 23, 1407–1412. [CrossRef] [PubMed]
541. Mahady, G.B. Is black cohosh estrogenic? Nutr. Rev. 2003, 61, 183–186. [PubMed]
542. Gaube, F.; Wolfl, S.; Pusch, L.; Kroll, T.C.; Hamburger, M. Gene expression profiling reveals effects of
Cimicifuga racemosa (L.) NUTT. (black cohosh) on the estrogen receptor positive human breast cancer cell line
MCF-7. BMC Pharmacol. 2007, 7, 11. [CrossRef] [PubMed]
543. Kennelly, E.J.; Baggett, S.; Nuntanakorn, P.; Ososki, A.L.; Mori, S.A.; Duke, J.; Coleton, M.; Kronenberg, F.
Analysis of thirteen populations of black cohosh for formononetin. Phytomedicine 2002, 9, 461–467. [CrossRef]
[PubMed]
544. Powers, C.N.; Setzer, W.N. A molecular docking study of phytochemical estrogen mimics from dietary
herbal supplements. Silico Pharmacol. 2015, 3, 4. [CrossRef] [PubMed]
545. Burdette, J.E.; Liu, J.; Chen, S.-N.; Fabricant, D.S.; Piersen, C.E.; Barker, E.L.; Pezzuto, J.M.; Mesecar, A.; van
Breemen, R.B.; Farnsworth, N.R.; et al. Black cohosh acts as a mixed competitive ligand and partial agonist
of the serotonin receptor. J. Agric. Food Chem. 2003, 51, 5661–5670. [CrossRef] [PubMed]
546. Rhyu, M.-R.; Lu, J.; Webster, D.E.; Fabricant, D.S.; Farnsworth, N.R.; Wang, Z.J. Black cohosh (Actaea racemosa,
Cimicifuga racemosa) behaves as a mixed competitive ligand and partial agonist at the human µ opiate
receptor. J. Agric. Food Chem. 2006, 54, 9852–9857. [CrossRef] [PubMed]
547. Reame, N.E.; Lukacs, J.L.; Padmanabhan, V.; Eyvazzadeh, A.D.; Smith, Y.R.; Zubieta, J.-K. Black cohosh has
central opioid activity in postmenopausal women: Evidence from naloxone blockade and PET neuroimaging
studies. Menopause 2008, 15, 832–849. [CrossRef] [PubMed]
548. Cicek, S.S.; Khom, S.; Taferner, B.; Hering, S.; Stuppner, H. Bioactivity-guided isolation of GABAA receptor
modulating constituents from the rhizomes of Actaea racemosa. J. Nat. Prod. 2010, 73, 2024–2028. [CrossRef]
[PubMed]
549. Borrelli, F.; Ernst, E. Cimicifuga racemosa: A systematic review of its clinical efficacy. Eur. J. Clin. Pharmacol.
2002, 58, 235–241. [CrossRef] [PubMed]
550. Borrelli, F.; Ernst, E. Black cohosh (Cimicifuga racemosa) for menopausal symptoms: A systematic review of
its efficacy. Pharmacol. Res. 2008, 58, 8–14. [CrossRef] [PubMed]
551. Frei-Kleiner, S.; Schaffner, W.; Rahlfs, V.W.; Bodmer, C.; Birkhäuser, M. Cimicifuga racemosa dried ethanolic
extract in menopausal disorders: A double-blind placebo-controlled clinical trial. Maturitas 2005, 51, 397–404.
[CrossRef] [PubMed]
552. Borrelli, F.; Ernst, E. Black cohosh (Cimicifuga racemosa): A systematic review of adverse events. Am. J. Obstet.
Gynecol. 2008, 199, 455–466. [CrossRef] [PubMed]
553. Schmid, D.; Woehs, F.; Svoboda, M.; Thalhammer, T.; Chiba, P.; Moeslinger, T. Aqueous extracts of
Cimicifuga racemosa and phenolcarboxylic constituents inhibit production of proinflammatory cytokines in
LPS-stimulated human whole blood. Can. J. Physiol. Pharmacol. 2009, 87, 963–972. [CrossRef] [PubMed]
554. Yang, C.L.H.; Chik, S.C.C.; Li, J.C.B.; Cheung, B.K.W.; Lau, A.S.Y. Identification of the bioactive constituent
and its mechanisms of action in mediating the anti-inflammatory effects of black cohosh and related
Cimicifuga species on human primary blood macrophages. J. Med. Chem. 2009, 52, 6707–6715. [CrossRef]
[PubMed]
555. Schmid, D.; Gruber, M.; Woehs, F.; Prinz, S.; Etzlstorfer, B.; Prucker, C.; Fuzzati, N.; Kopp, B.; Moeslinger, T.
Inhibition of inducible nitric oxide synthesis by Cimicifuga racemosa (Actaea racemosa, black cohosh) extracts
in LPS-stimulated RAW 264.7 macrophages. J. Pharm. Pharmacol. 2009, 61, 1089–1096. [CrossRef] [PubMed]
556. Erdelmeier, C.A.J.; Cinatl, J.; Rabenau, H.; Doerr, H.W.; Biber, A.; Koch, E. Antiviral and antiphlogistic
activities of Hamamelis virginiana bark. Planta Med. 1996, 62, 241–245. [CrossRef] [PubMed]
557. Duwiejua, M.; Zeitlin, I.J.; Waterman, P.G.; Gray, A.I. Anti-inflammatory activity of Polygonum bistorta,
Guaiacum officinale and Hamamelis virginiana in rats. J. Pharm. Pharmacol. 1994, 46, 286–290. [CrossRef]
[PubMed]
558. Hartisch, C.; Kolodziej, H.; von Bruchhousen, F. Dual inhibitory activities of tannins from Hamamelis
virginiana and related polyphenols on 5-lipoxygenase and lyso-PAF: Acetyl-CoA acetyltransferase. Planta
Med. 1997, 63, 106–110. [CrossRef] [PubMed]
559. Deters, A.; Dauer, A.; Schnetz, E.; Fartasch, M.; Hensel, A. High molecular compounds (polysaccharides and
proanthocyanidins) from Hamamelis virginiana bark: Influence on human skin keratinocyte proliferation and
differentiation and influence on irritated skin. Phytochemistry 2001, 58, 949–958. [CrossRef]
560. Theisen, L.L.; Erdelmeier, C.A.J.; Spoden, G.A.; Boukhallouk, F.; Sausy, A.; Florin, L.; Muller, C.P. Tannins
from Hamamelis virginiana bark extract: Characterization and improvement of the antiviral efficacy against
influenza A virus and human papillomavirus. PLoS ONE 2014, 9, e88062. [CrossRef] [PubMed]
561. Hughes-Formella, B.J.; Bohnsack, K.; Rippke, F.; Benner, G.; Rudolph, M.; Tausch, I.; Gassmueller, J.
Anti-inflammatory effect of Hamamelis lotion in a UVB erythema test. Dermatology 1998, 196, 316–322.
[CrossRef] [PubMed]
562. Dawid-Pa´c, R. Medicinal plants used in treatment of inflammatory skin diseases. Post˛ep. Dermatol. Alergol.
2013, 30, 170–177. [CrossRef] [PubMed]
563. Missouri Botanical Garden Tropicos. Available online: www.tropicos.org (accessed on 27 July 2018).
564. Memorial Sloan Kettering Cancer Center Goldenseal. Available online: www.mskcc.org (accessed on 16
October 2018).
565. Orfila, L.; Rodríguez, M.; Colman, T.; Hasegawa, M.; Merentes, E.; Arvelo, F. Structural modification of
berberine alkaloids in relation to cytotoxic activity in vitro. J. Ethnopharmacol. 2000, 71, 449–456. [CrossRef]
566. Cordero, C.P.; Gómez-González, S.; León-Acosta, C.J.; Morantes-Medina, S.J.; Aristizabal, F.A. Cytotoxic
activity of five compounds isolated from Colombian plants. Fitoterapia 2004, 75, 225–227. [CrossRef]
[PubMed]
567. Correché, E.R.; Andujar, S.A.; Kurdelas, R.R.; Lechón, M.J.G.; Freile, M.L.; Enriz, R.D. Antioxidant and
cytotoxic activities of canadine: Biological effects and structural aspects. Bioorganic Med. Chem. 2008, 16,
3641–3651. [CrossRef] [PubMed]
568. Kim, J.B.; Yu, J.-H.; Ko, E.; Lee, K.-W.; Song, A.K.; Park, S.Y.; Shin, I.; Han, W.; Noh, D.Y. The alkaloid
berberine inhibits the growth of Anoikis-resistant MCF-7 and MDA-MB-231 breast cancer cell lines by
inducing cell cycle arrest. Phytomedicine 2010, 17, 436–440. [CrossRef] [PubMed]
569. Mazzini, S.; Bellucci, M.C.; Mondelli, R. Mode of binding of the cytotoxic alkaloid berberine with the double
helix oligonucleotide d(AAGAATTCTT)2. Bioorganic Med. Chem. 2002, 11, 505–514. [CrossRef]
570. Kumar, G.S.; Das, S.; Bhadra, K.; Maiti, M. Protonated forms of poly[d(G-C)] and poly(dG).poly(dC) and
their interaction with berberine. Bioorganic Med. Chem. 2003, 11, 4861–4870. [CrossRef]
571. Ferraroni, M.; Bazzicalupi, C.; Bilia, A.R.; Gratteri, P. X-ray diffraction analyses of the natural isoquinoline
alkaloids berberine and sanguinarine complexed with double helix DNA d(CGTACG). Chem. Commun. 2011,
47, 4917–4919. [CrossRef] [PubMed]
572. Kuo, H.-P.; Chuang, T.-C.; Yeh, M.-H.; Hsu, S.-C.; Way, T.-D.; Chen, P.-Y.; Wang, S.S.; Chang, Y.-H.; Kao, M.-C.;
Liu, J.-Y. Growth suppression of HER2-overexpressing breast cancer cells by berberine via modulation of the
HER2/PI3K/Akt signaling pathway. J. Agric. Food Chem. 2011, 59, 8216–8224. [CrossRef] [PubMed]
573. Kuo, H.-P.; Chuang, T.-C.; Tsai, S.-C.; Tseng, H.-H.; Hsu, S.-C.; Chen, Y.-C.; Kuo, C.-L.; Kuo, Y.-H.; Liu, J.-Y.;
Kao, M.-C. Berberine, an isoquinoline alkaloid, inhibits the metastatic potential of breast cancer cells via Akt
pathway modulation. J. Agric. Food Chem. 2012, 60, 9649–9658. [CrossRef] [PubMed]
574. Iwasa, K.; Kamigauchi, M.; Ueki, M.; Taniguchi, M. Antibacterial activity and structure-activity relationships
of berberine analogs. Eur. J. Med. Chem. 1996, 31, 469–478. [CrossRef]
575. Kaneda, Y.; Torii, M.; Tanaka, T.; Aikawa, M. In vitro effects of berberine sulphate on the growth and structure
of Entamoeba histolytica, Giardia lamblia and Trichomonas vaginalis. Ann. Trop. Med. Parasitol. 1991, 85, 417–425.
[CrossRef] [PubMed]
576. Merschjohann, K.; Sporer, F.; Steverding, D.; Wink, M. In vitro effect of alkaloids on bloodstream forms of
Trypanosoma brucei and T. congolense. Planta Med. 2001, 67, 623–627. [CrossRef] [PubMed]
577. Vennerstrom, J.L.; Lovelace, J.K.; Waits, V.B.; Hanson, W.L.; Klayman, D.L. Berberine derivatives as
antileishmanial drugs. Antimicrob. Agents Chemother. 1990, 34, 918–921. [CrossRef] [PubMed]
578. Ropivia, J.; Derbré, S.; Rouger, C.; Pagniez, F.; Le Pape, P.; Richomme, P. Isoquinolines from the roots
of Thalictrum flavum L. and their evaluation as antiparasitic compounds. Molecules 2010, 15, 6476–6484.
[CrossRef] [PubMed]
579. Küpeli, E.; Ko¸sar, M.; Ye¸silada, E.; Ba¸ser, K.H.C.; Ba¸ser, C. A comparative study on the anti-inflammatory,
antinociceptive and antipyretic effects of isoquinoline alkaloids from the roots of Turkish Berberis species.
Life Sci. 2002, 72, 645–657. [CrossRef]
580. Mahady, G.B.; Chadwick, L.R. Goldenseal (Hydrastis canadensis): Is there enough scientific evidence to
support safety and efficacy? Nutr. Clin. Care 2001, 4, 243–249. [CrossRef]
581. Cicero, A.F.; Ertek, S. Metabolic and cardiovascular effects of berberine: From preclinical evidences to clinical
trial results. Clin. Lipidol. 2009, 4, 553–563. [CrossRef]
582. Hämet-Ahti, L. The Juncus effusus aggregate in eastern North America. Ann. Bot. Fenn. 1980, 17, 183–191.
583. Della Greca, M.; Fiorentino, A.; Molinaro, A.; Monaco, P.; Previtera, L. 9,10-Dihydrophenanthrene glucosides
from Juncus effusus. Nat. Prod. Lett. 1995, 6, 111–117. [CrossRef]
584. Park, S.N.; Won, D.H.; Hwang, J.P.; Han, S.B. Cellular protective effects of dehydroeffusol isolated from
Juncus effusus L. and the mechanisms underlying these effects. J. Ind. Eng. Chem. 2014, 20, 3046–3052.
[CrossRef]
585. Krochmal, A.; Walters, R.S.; Doughty, R.M. A Guide to Medicinal Plants of Appalachia; United States Department
of Agriculture: Upper Darby, PA, USA, 1969.
586. Nolan, J.M. The roots of tradition: Social ecology, cultural geography, and medicinal plant knowledge in the
Ozark-Ouachita Highlands. J. Ethnobiol. 1998, 18, 249–269.
587. Scholey, A.; Ossoukhova, A.; Owen, L.; Ibarra, A.; Pipingas, A.; He, K.; Roller, M.; Stough, C. Effects of
American ginseng (Panax quinquefolius) on neurocognitive function: An acute, randomised, double-blind,
placebo-controlled, crossover study. Psychopharmacology 2010, 212, 345–356. [CrossRef] [PubMed]
588. Barton, D.L.; Liu, H.; Dakhil, S.R.; Linquist, B.; Sloan, J.A.; Nichols, C.R.; McGinn, T.W.; Stella, P.J.;
Seeger, G.R.; Sood, A.; et al. Wisconsin ginseng (Panax quinquefolius) to improve cancer-related fatigue: A
randomized, double-blind trial, N07C2. J. Natl. Cancer Inst. 2013, 105, 1230–1238. [CrossRef] [PubMed]
589. McElhaney, J.E.; Goel, V.; Toane, B.; Hooten, J.; Shan, J.J. Efficacy of COLD-fX in the prevention of respiratory
symptoms in community-dwelling adults: A randomized, double-blinded, placebo controlled trial. J. Altern.
Complement. Med. 2006, 12, 153–157. [CrossRef] [PubMed]
590. McElhaney, J.E.; Simor, A.E.; McNeil, S.; Predy, G.N. Efficacy and safety of CVT-E002, a proprietary extract of
Panax quinquefolius in the prevention of respiratory infections in influenza-vaccinated community-dwelling
adults: A multicenter, randomized, double-blind, and placebo-controlled trial. Influenza Res. Treat. 2011, 2011.
[CrossRef] [PubMed]
591. Predny, M.L.; Chamberlain, J.L. Bloodroot (Sanguinaria canadensis) an Annotated Bibliography; U.S. Department
of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 2005.
592. Qing, Z.-X.; Yang, P.; Tang, Q.; Cheng, P.; Liu, X.-B.; Zheng, Y.; Liu, Y.-S.; Zeng, J.-G. Isoquinoline alkaloids
and their antiviral, antibacterial, and antifungal activities and structure-activity relationship. Curr. Org.
Chem. 2017, 21, 1920–1934. [CrossRef]
593. Obiang-Obounou, B.W.; Kang, O.-H.; Choi, J.-G.; Keum, J.-H.; Kim, S.-B.; Mun, S.-H.; Shin, D.-W.; Kim, K.W.;
Park, C.-B.; Kim, Y.-G.; et al. The mechanism of action of sanguinarine against methicillin-resistant
Staphylococcus aureus. J. Toxicol. Sci. 2011, 36, 277–283. [CrossRef] [PubMed]
594. Watamoto, T.; Egusa, H.; Sawase, T.; Yatani, H. Screening of pharmacologically active small molecule
compounds identifies antifungal agents against Candida biofilms. Front. Microbiol. 2015, 6, 1453. [CrossRef]
[PubMed]
595. Foster, S.; Duke, J.A. A Field Guide to Medicinal Plants; Houghton Mifflin: Boston, MA, USA, 1990.
596. Brock, C.; Whitehouse, J.; Tewfik, I.; Towell, T. The use of Scutellaria lateriflora: A pilot survey amongst herbal
medicine practitioners. J. Herb. Med. 2012, 2, 34–41. [CrossRef]
597. Zhu, J.T.T.; Choi, R.C.Y.; Li, J.; Xie, H.Q.H.; Bi, C.W.C.; Cheung, A.W.H.; Dong, T.T.X.; Jiang, Z.Y.; Chen, J.J.;
Tsim, K.W.K. Estrogenic and neuroprotective properties of scutellarin from Erigeron breviscapus: A drug
against postmenopausal symptoms and Alzheimer’s disease. Planta Med. 2009, 75, 1489–1493. [CrossRef]
[PubMed]
598. Liu, L.; Ma, H.; Tang, Y.; Chen, W.; Lu, Y.; Guo, J.; Duan, J.A. Discovery of estrogen receptor α modulators
from natural compounds in Si-Wu-Tang series decoctions using estrogen-responsive MCF-7 breast cancer
cells. Bioorganic Med. Chem. Lett. 2012, 22, 154–163. [CrossRef] [PubMed]
599. Liu, Y.F.; Gao, F.; Li, X.W.; Jia, R.H.; Meng, X.D.; Zhao, R.; Jing, Y.Y.; Wang, Y.; Jiang, W. The anticonvulsant
and neuroprotective effects of baicalin on pilocarpine-induced epileptic model in rats. Neurochem. Res. 2012,
37, 1670–1680. [CrossRef] [PubMed]
600. Park, H.G.; Yoon, S.Y.; Choi, J.Y.; Lee, G.S.; Choi, J.H.; Shin, C.Y.; Son, K.H.; Lee, Y.S.; Kim, W.K.; Ryu, J.H.; et al.
Anticonvulsant effect of wogonin isolated from Scutellaria baicalensis. Eur. J. Pharmacol. 2007, 574, 112–119.
[CrossRef] [PubMed]
601. Pan, Z.; Feng, T.; Shan, L.; Cai, B.; Chu, W.; Niu, H.; Lu, Y.; Yang, B. Scutellarin-induced
endothelium-independent relaxation in rat aorta. Phyther. Res. 2008, 22, 1428–1433. [CrossRef] [PubMed]
602. Yang, W.; Lust, R.M.; Bofferding, A.B.S.; Wingard, C.J. Nitric oxide and catalase-sensitive relaxation by
scutellarin in the mouse thoracic aorta. J. Cardiovasc. Pharmacol. 2009, 53, 66–76. [CrossRef] [PubMed]
603. Qu, J.T.; Zhang, D.X.; Liu, F.; Mao, H.P.; Ma, Y.K.; Yang, Y.; Li, C.X.; Qiu, L.Z.; Geng, X.; Zhang, J.M.; et al.
Vasodilatory effect of wogonin on the rat aorta and its mechanism study. Biol. Pharm. Bull. 2015, 38,
1873–1878. [CrossRef] [PubMed]
604. Shih, H.C.; Yang, L.L. Relaxant effect induced by wogonin from Scutellaria baicalensis on rat isolated uterine
smooth muscle. Pharm. Biol. 2012, 50, 760–765. [CrossRef] [PubMed]
605. Huang, Y.; Wong, C.M.; Lau, C.W.; Yao, X.; Tsang, S.Y.; Su, Y.L.; Chen, Z.Y. Inhibition of nitric oxide/cyclic
GMP-mediated relaxation by purified flavonoids, baicalin and baicalein, in rat aortic rings. Biochem.
Pharmacol. 2004, 67, 787–794. [CrossRef] [PubMed]
606. Liao, J.F.; Hung, W.Y.; Chen, C.F. Anxiolytic-like effects of baicalein and baicalin in the Vogel conflict test in
mice. Eur. J. Pharmacol. 2003, 464, 141–146. [CrossRef]
607. Hui, K.M.; Huen, M.S.Y.; Wang, H.Y.; Zheng, H.; Sigel, E.; Baur, R.; Ren, H.; Li, Z.W.; Wong, J.T.-F.; Xue, H.
Anxiolytic effect of wogonin, a benzodiazepine receptor ligand isolated from Scutellaria baicalensis Georgi.
Biochem. Pharmacol. 2002, 64, 1415–1424. [CrossRef]
608. Wang, F.; Xu, Z.; Ren, L.; Tsang, S.Y.; Xue, H. GABAA receptor subtype selectivity underlying selective
anxiolytic effect of baicalin. Neuropharmacology 2008, 55, 1231–1237. [CrossRef] [PubMed]
609. De Carvalho, R.S.M.; Duarte, F.S.; de Lima, T.C.M. Involvement of GABAergic non-benzodiazepine sites in
the anxiolytic-like and sedative effects of the flavonoid baicalein in mice. Behav. Brain Res. 2011, 221, 75–82.
[CrossRef] [PubMed]
610. Wolfson, P.; Hoffmann, D.L. An investigation into the efficacy of Scutellaria lateriflora in healthy volunteers.
Altern. Ther. Health Med. 2003, 9, 74–78. [PubMed]
611. Brock, C.A.; Whitehouse, J.; Tewfik, I.; Towell, T. American skullcap (Scutellaria lateriflora L.): A randomised,
double-blind placebo-controlled crossover study of its effects on mood in healthy volunteers. Phyther. Res.
2012, 28, 692–698. [CrossRef] [PubMed]
Copyright
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access
article distributed under the terms and conditions of the Creative Commons Attribution
(CC BY) license (http://creativecommons.org/licenses/by/4.0/)
Copyright © 2023 Farmers For Health Global Initiative - All Rights Reserved.
Powered by GoDaddy