From traditional Ayurvedic medicine to modern medicine
Identification of therapeutic targets for suppression of inflammation and cancer
Cancer is a hyperproliferative disorder that involves transformation, dysregulation of apoptosis, proliferation, invasion, angiogenesis and metastasis.
Extensive research during the last 30 years has revealed much about the biology of cancer. Drugs used to treat most cancers are those that can block cell signaling, including growth factor signalling (e.g., epidermal growth factor); prostaglandin production (e.g., COX-2); inflammation (e.g., inflammatory
cytokines: NF-κB, TNF, IL-1, IL-6, chemokines); drug resistance gene products (e.g., multi-drug resistance); cell cycle proteins (e.g., cyclin D1 and cyclin E); angiogenesis (e.g., vascular endothelial growth factor); invasion (e.g., matrix metalloproteinases); antiapoptosis (e.g., bcl-2, bcl-XL, XIAP, survivin, FLIP); and cellular proliferation (e.g., c-myc, AP-1, growth factors). Numerous reports have suggested that Ayurvedic plants and their components mediate their effects by modulating several of these recently identified therapeutic targets.
However, Ayurvedic medicine requires rediscovery in light of our current knowledge of allopathic (modern) medicine. The focus of this review is to elucidate the Ayurvedic concept of cancer, including its classification, causes, pathogenesis and prevention; surgical removal of tumours; herbal
remedies; dietary modifications; and spiritual treatments.

Introduction
According to the International Agency for Research on Cancer (IARC), in 2002, cancer killed > 6.7 million people around the world; another 10.9 million new cases were diagnosed; and at the current rate, an estimated 15 million people will be diagnosed annually by 2020. Cancer is one of the leading causes of death in the US and around the world. Several chemotherapeutic, cytotoxic, and immunomodulating agents are available in Western medicine to treat cancer. Besides being enormously expensive, these drugs are associated with severe side effects and morbidity. Still, the search continues for an ideal treatment that has minimal side effects and is cost-effective. Today, in Western medicine, only a limited number of plant products are being used to treat cancer. However, some of the widely used anticancer drugs, such as taxol and vinca alkaloids, are obtained from medicinal plants. This review focuses on the ancient perspective of cancer and how it can be integrated with modern science for the best treatment of cancer (Figure 1). Ayurveda, one of the significant traditional forms of medical practice in India, has produced many useful leads in developing medications for chronic diseases. Almost 25 centuries ago, Hippocrates proclaimed, ‘Let food be thy medicine and medicine be thy food.’ According to a recent report by Newman et al., as many as 65% of formally synthetic hypertension drugs are plant-based [1]. Of the 121 prescription drugs in use today for cancer treatment, 90 are derived from plants. Almost 74% of these, including taxol, was discovered by investigating a folklore claim [2,3]. Between 1981 and 2002, 48 out of 65 drugs approved for cancer treatment were natural products, based on natural products, or mimicked natural products in one form or another [1]. These phytochemicals are commonly called chemotherapeutic or chemopreventive agents.
Phytochemicals may fight disease through suppression of the inflammatory response. Dysregulated inflammation contributes to many diseases, including cancer [4,5]. It stands to reason then, that suppression of inflammation, whether by phytochemicals or other means, should delay the onset of disease [2,3]. Tumourigenesis is a multistep process that begins with cellular transformation, advances towards hyperproliferation, and culminates in the acquisition of invasive potential and angiogenic properties and the establishment of metastatic lesions [6]. This process can be activated by any of the various environmental carcinogens (such as cigarette smoke, industrial emissions, gasoline vapors), inflammatory agents (such as TNF and H 2O2), tumefaction promoters (such as phorbol esters and okadaic acid). This multistep process of carcinogenesis involves three phases: tumor initiation, promotion, and progression. Several population-based studies indicate that people in Southeast Asian countries have a much lower risk of developing colon, gastrointestinal, prostate, breast, and other cancers when compared with their Western counterparts.
It is likely that dietary constituents, such as garlic, ginger, soy, curcumin, onion, tomatoes, cruciferous vegetables, chilies, and green tea, play an essential role in protection from these cancers. These dietary agents are considered to halt the transformative, hyperproliferative, and inflammatory processes that initiate carcinogenesis. Their inhibitory properties may ultimately suppress the final steps of carcinogenesis as well, namely angiogenesis and metastasis.
These dietary constituents have been classified as chemopreventive agents, and their capacity to delay the onset of carcinogenesis has been studied extensively.
Because these chemopreventive agents are derived from natural sources, they are considered pharmacologically safe. The current review, although brief, evaluates the untapped therapeutic potential of these agents in the setting of several molecular targets that are currently under investigation.
Major objectives in cancer therapy
Within the last 50 years, significant advances have been made in our understanding of the underlying biology of cancer. One significant advance is the understanding that the suppression of specific cell signaling pathways can suppress tumorigenesis. These signaling pathways are discussed below.
1. Role of the NF-κB activation pathway in tumourigenesis
NF-κB is a family of closely related protein dimers that bind to a common sequence motif in DNA called the κB site [7]. The molecular identification of its p50 subunit (v-REL) as a member of the reticuloendotheliosis (REL) family of viruses provided the first evidence that NF-κB is linked to cancer. Research over the past decade has revealed that NF-κB is an inducible transcription factor for genes involved in cell survival, cell adhesion, inflammation, differentiation, and growth. In most resting cells, NF-κB is sequestered in the cytoplasm by binding to the inhibitory IκB proteins that block the nuclear localization sequences of NF-κB. NF-κB is activated by a variety of stimuli, such as carcinogens, inflammatory agents, and tumor promoters, including cigarette smoke, phorbol esters, okadaic acid, H2O2, and TNF. These stimuli promote dissociation of IκBα through phosphorylation, ubiquitinylation, and its ultimate degradation in the proteasomes. This process unmasks the nuclear localization sequence of NF-κB, facilitating its nuclear entry, binding to κB regulatory elements, and activation of transcription of target genes. Many of the target genes that are activated are critical to the establishment of the early and late stages of aggressive cancers, including expression of cyclin D1, apoptosis suppressor proteins such as bcl-2 and bcl-XL and those required for metastasis and angiogenesis, such as matrix metalloproteases (MMPs) and vascular endothelial growth factor (VEGF).
2. Role of the AP-1 activation pathway in cancer prevention
Activated protein-1 (AP-1) is another transcription factor that regulates the expression of several genes involved in cell differentiation and proliferation. Functional activation of the AP-1 transcription complex is implicated in tumor promotion as well as in malignant transformation. This complex consists of either homo- or heterodimers of the constituents of the JUN and FOS family of proteins [8]. This AP-1-mediated transcription of several target genes also can be activated by a complex network of signaling pathways that involve external signals such as growth factors, mitogen-activated protein kinases (MAPKs), extracellular signal-regulated protein kinases and c-jun N-terminal kinase (JNK). Some of the target genes activated by the AP-1 transcription complex mirror those activated by NF-κB and include cyclin D1, bcl-2, bcl-XL, VEGF, MMP, and urokinase plasminogen activator (uPA). Expression of genes such as MMP, and especially uPA, promotes angiogenesis and invasive growth of cancer cells. Most importantly, AP-1 can also improve the transition of tumor cells from an epithelial to a mesenchymal morphology, one of the early steps in tumor metastasis. These oncogenic properties of AP-1 are primarily dictated by the dimer composition of the AP-1 family proteins and their post-transcriptional and translational modifications.
3. Role of proliferation and apoptosis in tumourigenesis
Several reports have been published in the past eight years showing that activation of NF-κB promotes cell survival and proliferation, and downregulation of NF-κB sensitizes the cells to apoptosis. The mechanism through which NF-κB helps this proliferation, and cell survival mechanisms have become increasingly apparent. Expression of several genes, including bcl-2, bcl-XL, inhibitor-of-apoptosis protein (IAP), survivin, cyclin D1, TNF receptor-associated factor 1(TRAF1), and TRAF2, has been reported to be upregulated by NF-κB [9]. The proteins coded by these genes function primarily by blocking the apoptosis pathway. Several studies have demonstrated that NF-κB activation promotes cell survival and proliferation mechanisms and that suppression of NF-κB leads to abrogation of these mechanisms. Similarly, c-JUN is primarily a positive regulator of cell proliferation because c-jun-deficient fibroblasts have a marked proliferation defect in vitro and in vivo. c-jun protein, once fully activated by JNK kinases, induces transcription of the positive regulators of cell cycle progression, such as cyclin D1, and represses the negative regulators, such as the tumor suppressor p53 and the cyclin-dependent kinase inhibitor p16 (INK4A). Moreover, activated and oncogenic AP-1 can antagonize apoptosis in several tumors.
4. Growth factor activation pathway in tumourigenesis
The potent cell proliferation signals generated by various growth factor receptors, such as the epidermal growth factor receptor, insulin-like growth factor-1 receptor, and VEGF receptor networks, constitute the basis for receptor-driven tumorigenicity in the progression of several cancers [6]. The consequence of these abnormal growth factor receptor signaling pathways include increased cell proliferation, suppression of apoptotic signals (especially under anchorage-independent conditions), and an increase in the tumor’s invasive behavior, which contributes to metastatic spread and the growth of new blood vessels. Several chemopreventive phytochemicals, including curcumin, genistein, resveratrol, and catechins, recently are powerful inhibitors of several growth factor receptors, including epidermal growth factor receptor (EGFR). Some of these phytochemicals, such as curcumin, also can inhibit the ligand-stimulated activation of the EGFR, indicating that they have the potential to break the autocrine loops that are established in several advanced cancers [10]. The inhibitory actions of these phytochemicals have several other potential advantages in treating patients with late-stage cancers. A blockade of EGFR, for an example may predispose the cancer cells to apoptosis.
Moreover, inhibition of EGFR disables the protein’s capacity to provide the cancer cell the matrix-independent survival support it needs to expand and acquire invasive potential. Third, these chemopreventive chemicals function by inhibiting other tyrosine kinases, such as c-src, that are involved in the activation of the G-protein-coupled receptor to the transactivation of EGFR occurs extensively in established cancers. Finally, most of these phytochemicals also inhibit, by a similar mechanism, the HER2/neu receptor, which is overexpressed in breast, prostate, ovarian, and lung cancers. Curcumin has been shown not only to inhibit the tyrosine kinase activity of this receptor but also to deplete the protein itself. It does so by interfering with the function of the ATP-dependent GRP94 chaperone protein, which is involved in maintaining the properly folded state of the receptor [11].
Moreover, by inhibiting HER2/neu, most of these phytochemicals also can interfere with the cross-talk between the receptor and the estrogen receptor pathways in these cancers. Thus, they may be beneficial in treating hormone-resistant breast cancer patients by restoring their hormone responsiveness.
5. Role of the JAK–STAT pathway in tumourigenesis
Although cancer arises through several genetic or epigenetic mechanisms that contribute to several abnormal oncogenic signaling pathways, all seem to converge on a minimal number of nuclear transcription factors that function as final effectors, triggering specific gene expression patterns for particular cancer. These belong to the canonical signal transducers and activators of transcription (STAT) family of proteins [12]. They can be activated by phosphorylation through janus kinase (JAK) or cytokine receptors, G-protein-coupled receptors or growth factor receptors (such as EGFR); by platelet-derived growth factor receptors that have intrinsic tyrosine kinase activity; or by intracellular nonreceptor tyrosine kinase recruitment. Of the seven STAT proteins identified so far constitutive activations of STAT3 and STAT5 have been
implicated in multiple myeloma, lymphomas, leukemias and
several solid tumors, making these proteins logical targets for
cancer therapy. These STAT proteins contribute to cell survival and growth by preventing apoptosis through increased
expression of anti-apoptotic proteins, such as bcl-2 and
bcl-X
L. Recently, STAT 3 was shown to be a direct activator
of the VEGF gene, which is responsible for increased angiogenesis. More importantly, the increased expression of
STAT3 and STAT5 transcription factors are crucially
involved in the processes through which tumors evade
immunological surveillance by increasing the feeling of
immune-suppressing factors and decreasing the expression
of pro-inflammatory cytokines that are responsible for the
maturation of the dendritic cells [13].
6. Role of multi-drug resistance in tumourigenesis
MDR in human cancer is often associated with overexpression of the mdr-1 gene, which encodes a 170 kDa transmembrane protein, termed P-glycoprotein (P-gp). P-glycoprotein is considered to be of prognostic relevance in different tumor types. It is involved in resistance to natural product-based chemotherapeutics, including taxanes, anthracyclines, vinca alkaloids, podophyllotoxins, and camptothecins. Although several reports suggest that P-170 is clinically relevant in hematological malignancies, its role in solid tumors is not well understood. Its overexpression is correlated with the poor outcome observed in patients treated with chemotherapy and presenting drug resistance. Activation of the MDR-1 gene or selection of intrinsically MDR neoplastic cells may occur at the early stages of tumourigenesis of oral cancers before the real evidence of cellular transformation [14]. Thus, contact with possible chemical carcinogens, such as those of tobacco smoke, may induce activation of the MDR-1 gene. MDR-1 product expression in oral squamous cell carcinoma might suggest that overexpression of this protein could constitute a hallmark of potential more aggressive phenotype for this type of neoplasia. Quantitative flow-cytometric analysis of P-GP expression showed a significant increase in P-gp levels in untreated primary oral tumors and dysplastic lesions as compared with healthy oral tissues. A marked considerable improvement in P-GP expression was observed in recurrent oral carcinomas as compared with standard oral tissues and dysplastic lesions. Among recurrent tumors, a significant increase in the level of P-gp was observed in T4-stage tumors as compared with T3-stage tumors. Thus, P-gp is differentially expressed during oral tumourigenesis and might be an indicator of the biological behavior of oral malignancies [15]. Activation of MDR-related gene expression also occurs during the tumourigenesis of urothelial cancers and that it may confer de novo and acquired drug resistance on urothelial carcinomas [16]. Like cytochrome P450s (CYP3A4), P-gp is vulnerable to inhibition, activation, or induction by herbal constituents.
7. Role of COX-2 in tumourigenesis
Numerous preclinical studies point to the importance of regulating cyclooxygenase-2 (COX-2) expression in the prevention and, most importantly, to the treatment of several malignancies. This enzyme is overexpressed in practically every premalignant and malignant condition involving the colon, liver, pancreas, breast, lung, bladder, skin, stomach, head and neck, and esophagus [17]. COX-2 overexpression is a consequence of the deregulation of transcriptional and post-transcriptional control. Several growth factors, cytokines, oncogenes, and tumor promoters, stimulate COX-2 transcription. Expression of COX-2 is increased in HER2/neu-expressing breast carcinomas owing to enhanced ras signaling. Depending upon the stimulus and the cell type, different transcription factors, including AP-1, NF-IL-6, and NF-κB, can stimulate COX-2 transcription [17]. Wild-type p53 protein expression can suppress COX-2 transcription, whereas the mutant p53 protein cannot. Consistent with this observation, increased COX-2 levels are seen in several epithelial cancers that express mutant p53. Taken together, these findings suggest that the balance between the activation of oncogenes and the inactivation of tumor suppressor genes and the expression of several pro-inflammatory cytokines can modulate the expression of COX-2 in tumors. Complicating matters further is the fact that conventional cancer therapies, such as radiation and chemotherapy, can induce COX-2 and prostaglandin biosynthesis. Thus, inhibition of this enhanced COX-2 activity in tumors has therapeutic potential.
8. Role of angiogenesis in tumourigenesis
Angiogenesis, the regulated formation of new blood vessels from existing ones, is the basis of several physiological processes, such as embryonic development, placenta formation, and wound healing. It is one of the best examples of how a tumor can take control of these processes and deregulate them to its advantage. In the regular and orderly formation of new blood vessels, the endothelial cell receives the stimulatory signal and secretes MMP and heparanase, which causes the extracellular matrix to dissolve. The tight junction between the endothelial cells is then altered, and the cells project through the newly created space where freshly formed endothelial cells organize into fresh capillary tubes. This allows the sprouting vessel to grow toward the source of fresh blood [18]. When a tumor tries to build new blood vessels, most of these standard physiological rules governing new blood vessel growth are subverted. Blood vessels newly formed by tumors often have incomplete basement membranes, and the microvasculature is often chaotic, following convoluted paths without organization. These vessels also have a disproportionate ratio of endothelial cells to pericytes and abnormal pericyte coverage. The new blood vessels are hyperpermeable because of an imbalance of pro- and antiangiogenic factors, and they are often leaky [18]. Moreover, tumor cells themselves try to mimic the properties of endothelial cells and form a loose vasculogenic meshwork by processes such as vessel cooption and vasculogenic mimicry [19]. Thus, interference with the mechanisms of angiogenic switch, vessel cooption , and the vasculogenic imitation will be of high therapeutic value in several advanced cancers.
9. Role of cyclins in tumourigenesis
Hundreds of types of cancer exhibit global changes in gene expression, but only a minimal number of crucial alterations are common to all tumors. These common alterations are related to those that disrupt the normal cell cycle control checkpoints. The retinoblastoma and tumor suppressor p53 proteins that are crucial for these controls are usually lost in several cancers. The central role of the G1 to S and the G2 to M transitions and the corresponding checkpoints in cancer development are well established [20]. Formation and regulation of enzyme complexes with the D-type cyclins and their partners and the B-type cyclins with their associated proteins are particularly well characterized, as is the control of retinoblastoma function by phosphorylation.
Ayurvedic concept of cancer
Charaka and Sushruta Samhita (700 BC) both described the equivalent of cancer as granthi (benign or minor neoplasm) and arbuda (malignant or primary neoplasm) [21-23]. Both can be inflammatory or non-inflammatory, based on the doshas involved [24]. The term dosha describes the three principles that govern the psychophysiological response and pathological changes in the body. The balanced coordination of these three systems (Vata, Pitta, and Kapha) in body, mind, and consciousness is the Ayurvedic definition of health [25]. The fundamental theory of Ayurvedic treatment is based on the restoration of the balance between these three major bodily systems. Tridoshic tumors are usually malignant because all three dominant body humors lose mutual coordination, resulting in a morbid condition [26,27]. Ayurvedic classification of neoplasms depends upon various clinical symptoms concerning tridoshas.
- Group I: Diseases that can be named as apparent malignancies, including arbuda and granthi, such as mamsarbuda (sarcomas) and raktarbuda (leukemia), mukharbuda (oral cancer), and asadhya Vrana (acute or malignant ulcers).
- Group II: Diseases that can be considered as cancer or probable malignancies, such as ulcers and growths. Examples of these are mamsaja oshtharoga (growth of lips), asadhya galganda (incurable thyroid tumor), tridosaja gulmas, asadhya udara roga, (abdominal tumors like carcinomas of the stomach and liver or lymphomas).
- Group III: Diseases with the possibility of malignancy, such as visarpa (erysipelas), asadhya Kamala (incurable jaundice), asadhya pradara (intractable leukorrhea) and tridosaja Nadi Vrana (indomitable sinusitis).
Source of anticancer drugs from Ayurvedic medicine
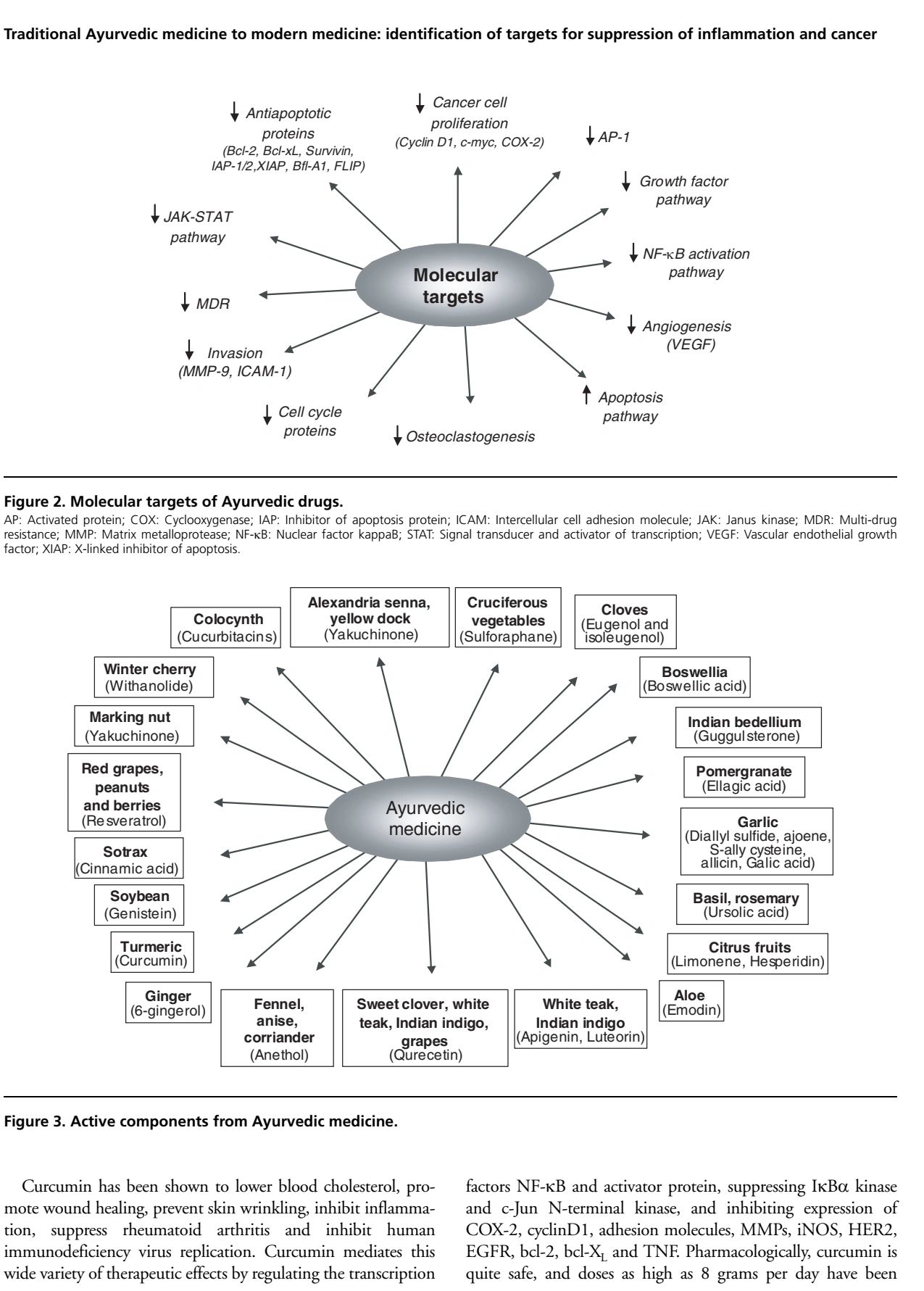
Some of the herbs commonly used in Ayurveda are listed in Table 1 and Figures 3, 4 and 5.
The active components of these
herbs, which have anticancer activity, and their molecular targets are described below (Tables 2, 3 and 4, Figures 2 and 3).
1. Guggulsterone (Commiphora mukul)
1. Guggulsterone (Commiphora mukul)
1. Guggulsterone (Commiphora mukul)

Guggulsterone [4,17(20)-pregnadiene-3,16-dione] is a plant sterol derived from the gum resin (Guggulu) of the tree Commiphora Mukul. The resin has been used in Ayurvedic medicine for centuries to treat a variety of ailments, including obesity, bone fractures, arthritis, inflammation, cardiovascular disease, and lipid disorders [28,29]. The antiarthritic and anti-inflammatory activities of gum guggul were demonstrated as early as 1960 by Gujral et al. [30]. Sharma et al. showed guggul’s activity in experimental arthritis induced by a mycobacterial adjuvant [31]. The effectiveness of guggul for treating osteoarthritis of the knee also has been demonstrated [32]. Recent studies have shown that guggulsterone is an antagonist for the bile acid receptor farnesoid X receptor [33,34]. Other studies have shown that guggulsterone enhances transcription of the bile salt export pump [35], thereby regulating cholesterol homeostasis. An understanding of the molecular mechanisms underlying guggulsterone is just now emerging. In 2003, Meselhy et al. showed that guggulsterone could suppress inflammation by inhibiting inducible nitric oxide synthetase (iNOS) expression induced by lipopolysaccharide in macrophages [36]. Because most inflammatory diseases are mediated through the activation of NF-κB, a nuclear transcription factor [7,37], the authors hypothesize that it is involved in guggulsterone’s activity. Guggulsterone suppresses DNA binding of NF-κB induced by TNF, phorbol ester, okadaic acid, cigarette smoke condensate, hydrogen peroxide, and IL-1. Guggulsterone also suppressed the constitutive NF-κB activation expressed in most tumor cells. Besides, guggulsterone decreases the expression of gene products involved in antiapoptosis (IAP1), X chromosome-linked IAP, Bfl-1/A1, bcl-2, cFLIP and survivin), proliferative genes (cyclin D1, c-myc), and metastatic genes (MMP-9, COX-2, and VEGF). This correlated with the enhanced apoptosis induced by TNF and chemotherapeutic agents [38].
2. Curcumin (Curcuma longa)
1. Guggulsterone (Commiphora mukul)
1. Guggulsterone (Commiphora mukul)

Curcumin (diferuloylmethane) is an active component of turmeric (Curcuma longa), which has been used as a spice and as an Ayurvedic medicine for centuries on the Indian subcontinent. Curcumin has been shown to suppress carcinogenesis of the skin, liver, lung, colon, stomach, and breast. It has also been shown to inhibit the proliferation of a wide variety of tumor cells in culture and to promote apoptosis through Bid cleavage, cytochrome c release, caspase-9 activation and then caspase-3 activation [39-60]. Curcumin has been shown to lower blood cholesterol, promote wound healing, prevent skin wrinkling, inhibit inflammation, suppress rheumatoid arthritis, and inhibit human immunodeficiency virus replication. Curcumin mediates this wide variety of therapeutic effects by regulating the transcription factors NF-κB and activator protein, suppressing IκBα kinase and c-Jun N-terminal kinase, and inhibiting expression of COX-2, cyclinD1, adhesion molecules, MMPs, iNOS, HER2, EGFR, bcl-2, bcl-XL, and TNF. Pharmacologically, curcumin is quite safe, and doses as high as 8 grams per day have been administered orally to humans with no side effects. The numerous therapeutic activities of curcumin, its pharmacological safety, and its color qualify it as ‘Indian solid gold.’ Extensive research over the last 50 years has indicated that curcumin can both prevent and treat cancer. Curcumin’s anti-cancer potential stems from its ability to suppress the proliferation of a wide variety of tumor cells; downregulate transcription factors NF-κB, AP-1, and Egr-1; downregulate the expression of COX-2, LOX, iNOS, MMP-9, uPA, TNF, chemokines, cell surface adhesion molecules, and cyclin D1; downregulate growth factor receptors (such as EGFR and HER2); and inhibit the activity of c-Jun N-terminal kinase, protein tyrosine kinases, and protein serine/threonine kinases. In several systems, curcumin is a potent antioxidant and anti-inflammatory agent.
Further evidence suggests that curcumin can suppress tumor initiation, promotion, and metastasis. Human clinical trials have indicated no dose-limiting toxicity when curcumin is administered at doses up to 10 grams per day. All these studies suggest that curcumin has enormous potential in the prevention and treatment of cancer.

3. Resveratrol (Vitis vinifera)
The history of resveratrol can be traced back thousands of years. Perhaps the first known use of grape extracts for human health occurred > 2000 years ago in ‘darakchasava,’ a well-known Indian herbal preparation whose main ingredient is Vitis vinifera L. This ‘Ayurvedic’ medicine is prescribed as a cardiotonic and also is given for other disorders [61]. The use of dried grapes (also called manakka) as a cardiotonic is well documented. High-performance liquid chromatography analysis of darakchasava revealed the presence of polyphenols, such as resveratrol and pterostilbene. Interest in this age-old formulation grew in light of this new knowledge of resveratrol. Resveratrol, trans-3,5,4′-trihydroxy stilbene, was first isolated in 1940 as a constituent of the roots of white hellebore (Veratrum grandiflorum O. Loes) but has since been found in various plants, including grapes, berries, and peanuts [62-65]. Besides cardioprotective effects, resveratrol exhibits anticancer properties, as suggested by its ability to suppress proliferation of a wide variety of tumor cells, including lymphoid and myeloid cancers, multiple myeloma, cancers of the breast, prostate, stomach, colon, pancreas, and thyroid, melanoma, head, and neck squamous cell carcinomas, ovarian carcinoma and cervical carcinoma. The growth-inhibitory effects of resveratrol are mediated through cell-cycle arrest, upregulation of p21Cip1/WAF1, p53 and Bax, downregulation of survivin, cyclin D1, cyclin E, bcl-2, bcl-XL, and cIAPs, and activation of caspases. Resveratrol has been shown to suppress the activation of several transcription factors, including NF-κB, AP-1, and Egr-1; inhibit protein kinases, including IκBα kinase, JNK, MAPK, Akt, PKC, PKD, and casein kinase II; and downregulate products of genes such as COX-2, 5-lipoxygenase (5-LOX), VEGF, IL-1, IL-6, IL-8,
androgen receptor, and, prostate-specific antigen. These activities account for this stilbene’s suppression of angiogenesis. Resveratrol also has been shown to potentiate the apoptotic effects of cytokines (such as TRAIL, chemotherapeutic agents and γ-radiation. Pharmacokinetic studies have revealed that resveratrol’s target organs are liver and kidney, where it is concentrated after absorption and is mainly converted to a sulfated form and a glucuronide conjugate. In vivo, resveratrol blocks the multistep process of carcinogenesis at various stages: It blocks carcinogen activation by inhibiting aryl hydrocarbon-induced CYP1A1 expression and activity and suppresses tumor initiation, promotion, and progression. Besides chemopreventive effects, resveratrol appears to exhibit therapeutic effects against cancer. Limited data in humans have revealed that resveratrol is pharmacologically quite safe. Currently, structural analogs of resveratrol with improved bioavailability are being pursued as potential therapeutic agents for cancer.
4.Flavopiridol (Dysoxylum binectariferum)
4.Flavopiridol (Dysoxylum binectariferum)
4.Flavopiridol (Dysoxylum binectariferum)

Flavopiridol is a semisynthetic flavonoid closely related to a compound originally isolated from the stem bark of Dysoxylum binectariferum (also called rohitukine from Amoora rohituka), a plant indigenous to India and described in Ayurveda. The parent compound is identical to flavopiridol except that a methyl group replaces the chlorophenyl moiety at position 2. Flavopiridol is a potent inhibitor of cyclin-dependent kinase (CDK) 1, CDK 2, CDK 4 and CDK 7 [66]. It inhibits CDKs by competing with adenosine triphosphate at the nucleotide-binding site on CDKs, as indicated by kinetics studies [67] and X-ray crystallography of the CDK 2–flavopiridol complex [68]. The tyrosine phosphorylation of CDK 2 is also inhibited by this flavone[69]. Through inhibition of CDKs, flavopiridol induces arrest of cell growth at the G1 and G2 phases of the cell cycle [66,70]. Because of its ability to suppress the growth of breast carcinoma [66], lung carcinoma [71], chronic B cell leukemia and lymphoma [72-74], multiple myeloma [75] and head and neck squamous cell carcinoma [76], flavopiridol is currently in clinical trials for the treatment of several cancers [77-79]. Flavopiridol also has been shown to enhance the activity of other growth-suppressing agents, such as TNF, doxorubicin, and etoposide [80-84]. Flavopiridol also inhibits CDKs, induces apoptosis, suppresses inflammation, and modulates the immune response. Flavopiridol suppressed TNF activation of NF-κB in a dose- and time-dependent manner in several cell types, with optimal inhibition occurring when cells were treated with 100 nM of flavopiridol for 6 h [85].
5. Zerumbone (Zingiber zerumbet Smith)
4.Flavopiridol (Dysoxylum binectariferum)
4.Flavopiridol (Dysoxylum binectariferum)

Zerumbone (2,6,9,9-tetramethyl-[2E,6E,10E]-cycloundeca- 2,6,10-tried-1-one) was first isolated in 1956 from the essential oil of the rhizomes of wild ginger, Zingiber zerumbet Smith, which is widespread in Southeast Asia [86,87]. Over the years, a wide variety of activities have been ascribed to this compound [88-94]. For instance, zerumbone has been found to suppress the proliferation of colon cancer [93,94] and breast cancer [93], with minimal effects on normal cells [94]. Zerumbone also has been shown to suppress inflammation [92], suppress the initiation and promotion of skin tumors in mice [91], and prevent azoxymethane-induced aberrant crypt foci formation in rats [90]. Besides, this terpenoid has been shown to suppress dextran sodium sulfate-induced colitis in mice [95] and to inhibit the activation of the phorbol ester-induced Epstein-Barr virus [88]. Zerumbone also has been found to suppress superoxide and nitric oxide generation [89] and downregulate COX-2 [96], IL-1β [95] and TNF [94,95]. A potential explanation for several of these activities is that zerumbone may downregulate NF-κB activation [97]. The authors’ laboratory has shown that zerumbone suppressed NF-κB activation induced by a variety of agents. Interestingly, α-humulene, a structural analog of zerumbone lacking the carbonyl group, was completely inactive. Besides being inducible, constitutively active NF-κB also was inhibited. This downregulation potentiated apoptosis induced by cytokines and chemotherapeutic agents. Zerumbone’s inhibition of the expression of these NF-κB-regulated genes also correlated with the suppression of TNF-induced invasion activity. Overall, this inhibition may provide a molecular basis for exploring zerumbone’s potential in the prevention and treatment of cancer.
6. Withanolide (Withania sominifera)
7. Boswellic acid (Boswellia serrata)
7. Boswellic acid (Boswellia serrata)

The medicinal plant Withania somnifera is widely known for its anti-inflammatory, cardioactive and CNS effects. In Ayurveda, withanolide, which is extracted from W. somnifera, is employed in the treatment of arthritis and menstrual disorders and is known to be potent inhibitors of angiogenesis, inflammation, tumor development, metastasis and oxidative stress, and a promoter of cardioprotection. Many pharmacological studies have investigated the properties of W. foraminifera in an attempt to authenticate its use as a multipurpose medical agent. Experimental studies have shown that W. foraminifera possesses anti-inflammatory, antitumor, cardioprotective, and antioxidant properties. Withaferin A, one of the compounds in the withanolide family, is a potent inhibitor of angiogenesis. It also appears to exert a positive influence on the endocrine, urogenital, and central nervous systems. In recent years, herbal formulations containing substantial amounts of W. foraminifera root extract have been evaluated in small clinical trials and shown to have efficacy in the treatment of osteoarthritis. Excepts are also known to inhibit tumor growth in vivo significantly. However, the mechanisms responsible for the antitumor effects of withanolide are still unknown. The authors found that withanolide suppressed NF-κB activation induced by a wide variety of inflammatory and carcinogenic agents, including TNF, IL-1β, doxorubicin, and cigarette smoke condensate. Withanolide also enhanced the apoptosis induced by TNF and chemotherapeutic agents and suppressed invasion. These results indicate that withanolide inhibits activation of NF-κB and NF-κB-regulated gene expression. This may explain their ability to enhance apoptosis and inhibit invasion.
7. Boswellic acid (Boswellia serrata)
7. Boswellic acid (Boswellia serrata)
7. Boswellic acid (Boswellia serrata)

Boswellic acid (BA) is an active component of Boswellia serrata (also known as Salai guggul). The gum-resin of this plant is used in Ayurvedic medicine to treat rheumatic diseases, respiratory diseases, and liver disorders [98-100]. Extensive research within the last 30 years has identified the active component of this resin as BA (a pentacyclic triterpenic acid) and its derivatives (acetyl-β-boswellic acid, 11-keto-β-boswellic acid and acetyl-11-keto-β-boswellic acid [AKBA]) [101,102]. The traditional therapeutic usefulness of BA is a result of its anti-inflammatory activity, possibly mediated through the inhibition of 5-LOX [102-104] and leukocyte elastase [105,106]. In animal models of inflammation, BA is effective against Crohn’s disease, ulcerative colitis, and ileitis [107-109]; adjuvant or BSA-induced arthritis [110,111]; galactosamine/endotoxin-induced hepatitis in mice [112]; and osteoarthritis [113]. BA has antitumor effects in addition to its anti-inflammatory effects. It has been found to have activity against brain tumors [114,115], leukemic cells [116,117], colon cancer cells [118], metastatic melanoma and fibrosarcoma cells [119], and hepatoma [118]. BA also has been shown to inhibit the azoxymethane-induced formation of aberrant crypt foci in the colon of mice [120]. AKBA, a component of Boswellia serrata, is a pentacyclic terpenoid that is active against numerous inflammatory diseases, including cancer, arthritis, chronic colitis, ulcerative colitis, Crohn’s disease, and bronchial asthma. The authors found that AKBA potentiates the apoptosis induced by TNF and chemotherapeutic agents suppress TNF-induced invasion and inhibits the receptor activator of NF-κB ligand-induced osteoclastogenesis, all of which are known to require NF-κB activation (Takada et al., unpublished observations, 2005).
8. Fruits and vegetables

Fruits and vegetables are an integral part of Ayurvedic medicine. Steinmetz and Potter reviewed the scientific literature on the relationship between vegetable and fruit consumption and the risk of cancer [121]. After reviewing results from 206 human epidemiological studies and 22 animal studies, they found clear evidence that a higher intake of vegetables and fruits protect against cancers of the stomach, esophagus, lung, oral cavity and pharynx, endometrium, pancreas and colon. The types of vegetables and fruits that most often appear to be protective against cancer are raw vegetables, followed by cooked allium vegetables, carrots, green vegetables, cruciferous vegetables and tomatoes. The substances in vegetables and fruits that may help protect against cancer include dithiolthiones, isothiocyanates, indole-3-carbinol (I3C), allium compounds, isoflavones, protease inhibitors, saponins, phytosterols, inositol hexaphosphate, vitamin C, d-limonene, lutein, folic acid, β-carotene, lycopene, selenium, vitamin E and dietary fiber. How fruits and vegetables mediate their effects is beginning to be revealed [122,123]. For instance, I3C is produced by members of the family Cruciferae, and particularly members of the genus Brassica (for example, cabbage, radishes, cauliflower, broccoli, Brussels sprouts, and daikon). Under acidic conditions, I3C is converted to a series of oligomeric products (among which 3,3’-diindolylmethane is a significant component) believed to be responsible for its biological effects in vivo. In vitro, I3C has been shown to suppress the proliferation of various tumor cells, including those from breast, prostate, endometrial, and colon cancers and leukemia; induce G1/S cell-cycle arrest, and induce apoptosis. The cell-cycle arrest involves downregulation of cyclin D1, cyclin E, CDK2, CDK4 and CDK6 and upregulation of p15, p21, and p27. Apoptosis by I3C involves downregulation of antiapoptotic gene products, including bcl-2, bcl-XL, survivin, IAP, X-linked inhibitor of apoptosis, and Fas-associated death domain protein-like IL-1-β-converting enzyme inhibitory protein (FLIP), upregulation of pro-apoptotic protein bax, the release of mitochondrial cytochrome c, and activation of caspase-9 and caspase-3. This agent inhibits the activation of various transcription factors, including NF-κB, SP1, estrogen receptor, androgen receptor, and nuclear factor-E2-related factor 2. This indole potentiates the effects of TRAIL through induction of death receptors and synergizes with chemotherapeutic agents through the downregulation of P-gp. In vivo, I3C was found to be a potent chemopreventive agent for hormone-dependent cancers, such as breast and cervical cancers. These effects are mediated through its ability to induce apoptosis, inhibit DNA-carcinogen adduct formation, suppress free radical production, stimulate 2-hydroxylation of estradiol, and inhibit invasion and angiogenesis. Numerous studies have indicated that I3C also has intense hepatoprotective activity against various carcinogens. Initial clinical trials in women have shown that I3C is a promising agent against breast and cervical cancers.
Ayurvedic agents as chemosensitisers and radiosensitisers
The resistance of tumors to radiation and chemotherapeutic agents is collective, but no drug has yet been approved to overcome this chemoresistance or radioresistance. Although hydroxyurea, 5-fluorouracil, and cisplatin are currently used for radiosensitization, they are highly toxic. Recent reports point out that the safe and non-toxic agents described in Ayurvedic medicine can function as sensitizers, augmenting the effectiveness of cancer chemotherapy and radiotherapy [124]. For instance, plumbagin, derived from the plant Plumbago zeylanica, has been reported to enhance the effect of radiation in mice bearing sarcoma S180 and Ehrlich ascites carcinoma [125]. Tetrandrine (from the root of Stephenia tetrandra), withaferin-A (from Withania somnifera), echitamine chloride (from stem the bark of Astonia scholaris), rohitukine (from Amoora rohituka); curcumin (from Curcuma longa), and perillyl alcohol and berberine (from Tinospora cordifolia) have been shown to possess radiosensitizing activities in vitro and in vivo [126-135]. This sensitization is believed to occur at various levels. First, by directly competing with the ATP binding site of the multi-drug resistance (MDR) or multidrug resistance-associated protein (MRP) drug efflux pumps, curcumin can inhibit the pump and increase intracellular concentrations of chemotherapeutic drugs, such as vinblastine or vincristine. Second, by functioning as efflux substrates for pumps, such as MDR or MRP, chemopreventive agents such as genistein and green tea components ( (-)-epigallocatechin-3-gallate [EGCG]) can saturate and hence titrate out the pumps, increasing the amount of chemotherapeutic drug within the cell. This type of competition with the MDR or MRP substrates in effect sensitize the cancer cell for a better cell kill by chemotherapeutic agents. Third, curcumin can interfere with the functioning of pumps such as MRP, that require a a steady supply of reduced glutathione GSH because it is a known inhibitor of GSH synthetase. This type of inhibition might enhance the sensitivity of cancer cells that overexpress MRP to chemotherapeutic agents such as vincristine, arsenicals and platinum derivatives by impairing their efflux [136]. Another clinical strategy that is currently being pursued is that of targeting c-JUN expression to reduce intracellularly GSH levels. Stable increases in the c-JUN expression are associated with an AP-1–mediated increase in GSH synthetase levels [137]. Because curcumin targets the same elements, it would be a potent inhibitor, reducing intracellular GSH at the transcriptional level [138]. Expression of glutathione S-transferase Pi (GST-Pi) also is associated with cancer cells’ resistance to chemotherapeutic agents. In a recent study, curcumin efficiently inhibited the TNF- and phorbol ester-induced AP-1 and NF-κB transcription factor binding to the sites located on the GST-Pi gene promoter in K562 leukemia cells [138]. This process efficiently reduced GST-Pi levels, interfering with drug resistance, and, ultimately, with apoptosis. Chemopreventive agents such as curcumin also can sensitize cancer cells to other traditional chemotherapeutic agents such as etoposide and camptothecin in another capacity. Topo-II poisons stabilize the cleavable complexes, an intermediate product of the TopoII-catalysed reaction. Accumulation of these cleavable complexes is believed to lead to cell death. Conversely, a decrease in the number of cleavable compounds could confer drug resistance. Proteasome inhibition was recently found to decrease this inducible resistance by inhibiting the Topo-II depletion by hypoxia or glucose starvation.
Moreover, the observation that proteasomal inhibitors, such as lactacystin, significantly enhance the antitumor activity of etoposide in xenografts in vivo strongly suggests that the Topo-II depletion occurs through a proteasomal mechanism [139]. Following this rationale, several proteasomal inhibitors, such as PS-341, are currently showing promise in Phase II clinical trials. It is worth noting that curcumin has recently been shown to inhibit cellular proteasome activity in a concentration-dependent manner with a parallel increase in the accumulation of ubiquitinated proteins. This agent may be able to inhibit the proteasomes by inhibiting ubiquitin isopeptidase activity, as shown in recent studies [140]. Curcumin’s proteasome-mediated sensitization of cancer cells to drugs such as etoposide and camptothecin would be beneficial in the treatment of several types of cancer. This expectation is based on proteasomes’ inhibition of Topo-II degradation, which would result in more DNA cleavable complexes. Most of the chemotherapeutic agents and γ irradiation commonly administered to cancer patients activate NF-κB. NF-κB activation can lead to resistance to apoptosis. Activation of these survival processes occurs in parallel with the induction of apoptosis through the same agents’ activation of several caspases. In this respect, co-administration of chemopreventive agents such as curcumin would activate apoptotic pathways while downregulating cell survival pathways mediated by phosphoinositol-3 kinase and Akt proteins. This generally can be accomplished without activating the anti-apoptotic pathways that, in effect, alter the bcl-2: bax ratio and contribute to the sensitizing effect. The sensitizing or potentiating results of these chemopreventives would then allow cancer treatments to achieve a better target cell kill than what can be achieved by chemotherapy or radiotherapy alone. Other mechanisms by which curcumin and other chemopreventive agents may enhance the cytotoxicity of chemo- and radiotherapies include the induction of p21WAF-1/CIP1. Recently, resveratrol was found to mediate chemosensitization through the downregulation of survivin, a cell survival gene [141]. Similarly, curcumin was found to induce the radiosensitization of prostate cancer cells through suppression of NF-κB activation [128]. Study of antitumor and radiosensitizing properties of Withania somnifera (Ashwagandha), a well known medicinal plant, have yielded encouraging results [142]. The alcoholic extract of the dried roots of the plant as well as the active component withaferin A isolated from the extract showed significant antitumor and radiosensitizing effects in experimental tumors in vivo, without any noticeable systemic toxicity Withaferin A gave a sensitizer enhancement ratio of 1.5 for in vitro cell killing of V79 Chinese hamster cells at a non-toxic concentration of ∼ 2 µM. Although the mechanism of action of this compound is not known, the studies so far indicate that W. somnifera could prove to be an excellent natural source of a potent and relatively safe radiosensitizer/chemotherapeutic agent. Similarly, the fruit pulp of Emblica Officinalis (EO) is an important drug used in Indian systems of medicine for several diseases and as a tonic. Given its diverse uses, the aqueous plant extract was tested for its radioprotective properties against sublethal γ-radiation (9 Gy) in Swiss albino mice [143]. Animals were divided into two groups and irradiated with γ-radiation externally, with or without EO extract, which was given orally at different doses before irradiation. The dose of fruit pulp extract found to be most effective against radiation was 100 mg/kg b.wt. This dose increased the survival time and reduced the mortality rate of mice significantly. Furthermore, body weight loss in EO administered irradiated animals was considerably less in comparison with animals who were given radiation only. In general, these chemopreventive agents achieve significant sensitization by overcoming therapy-induced pro-survival gene expression in several cancers.
Herb–drug interactions
The present interest and widespread use of herbal remedies have created the possibility of interaction between them and pharmaceutical drugs if they are used simultaneously. A wide variety of phenolic compounds and flavonoids present in spices have been tested for their effects on 5-LOX, the key enzyme involved in the biosynthesis of leukotrienes [144]. All these compounds significantly inhibited the formation of lipoxygenase in a concentration-dependent manner, and the combinations of spice active principles/extracts exerted a synergistic effect in inhibiting 5-LOX activity. P-gp is responsible for the systemic disposition of numerous structurally and pharmacologically unrelated lipophilic and amphipathic drugs, carcinogens, toxins, and other xenobiotics in many organs, such as the intestine, liver, kidney, and brain. P-gp is vulnerable to inhibition, activation, or induction by herbal constituents. Curcumin, ginsenosides, piperine, some catechins from green tea, and silymarin from milk thistle were found to be inhibitors of P-gps. In contrast, some catechins from green tea increased P-gpss-mediated drug transport by heterotropic allosteric mechanism, and St. John’s wort induced the intestinal expression of P-gps in vitro and in vivo. Some components (e.g., bergamottin and quercetin) from grapefruit juice were reported to modulate P-gp activity. The inhibition of P-gp by herbal constituents may provide a novel approach for reversing MDR in tumor cells, whereas the stimulation of P-gp expression or activity implies chemoprotective enhancement by herbal medicines. Certain natural flavonols (e.g., kaempferol, quercetin, and galangin) are potent stimulators of the P-gp-mediated efflux of 7,12-dimethylbenz(a)- anthracene (a carcinogen). The modulation of P-gp activity and expression by these herb constituents may result in altered absorption and bioavailability of drugs that are P-gp substrates. This is exemplified by increased oral bioavailability of phenytoin and rifampin by piperine and decreased bioavailability of indinavir, tacrolimus, cyclosporin, digoxin, and fexofenadine by coadministered St. John’s wort [145]. The medicinal properties of curcumin are limited because of poor bioavailability due to its rapid metabolism in the liver and intestinal wall. When curcumin was administered with piperine, the bioavailability was increased by 154% in rats. On the other hand, in humans, after a dose of 2 g curcumin alone, serum levels were either undetectable or very low. Concomitant administration of piperine increased in bioavailability by 2000% [146]. The study shows that piperine enhances the serum concentration, extent of absorption, and bioavailability of curcumin in both rats and humans with no adverse effects. EGCG, cotreatment with piperine (from black pepper), enhanced the bioavailability of EGCG in mice [147]. These studies demonstrated the modulation of bioavailability by a second dietary component and illustrate a mechanism for interactions between nutritional chemicals. Grapefruit juice has been shown to interact with certain drugs. The co-administration of these drugs with grapefruit juice can markedly elevate drug bioavailability and can alter pharmacokinetic and pharmacodynamic parameters of the drug. The predominant mechanism for this interaction is the inhibition of cytochrome P450 (CYP) 3A4 in the small intestine, resulting in a significant reduction of drug presystemic metabolism. An additional mechanism is inhibition of P-gp, a transporter that carries drugs from the enterocyte back to the gut lumen, resulting in a further increase in the fraction of drug absorbed. Some calcium channel antagonists, benzodiazepines, HMG-CoA reductase inhibitors, and cyclosporin are the most affected drugs [148]. Bergamottin, a furocoumarin derivative from grapefruit juice shows an inhibitory effect on simvastatin metabolism [149]. Even one glass of grapefruit juice, taken daily, considerably increases the plasma concentrations of simvastatin and simvastatin acid. Grapefruit juice may, thus, increase both the cholesterol-lowering effect and the risk of adverse effects of simvastatin [150]. In another study, Lilja et al. had shown that when simvastatin was taken with grapefruit juice, the mean peak serum concentration of simvastatin was increased 12.0-fold, compared with control. When simvastatin was administered 24 h after ingestion of the last dose of grapefruit juice, the peak serum concentration was increased 2.4-fold compared with control. When simvastatin was given three days after the intake of grapefruit juice, the peak serum concentration was increased 1.5-fold compared with control. Seven days after the ingestion of grapefruit juice, no differences in the peak serum concentration of simvastatin in comparison to control were seen. The interaction potential of even high amounts of grapefruit juice with CYP3A4 substrates dissipates within 3 – 7 days after ingestion of the last dose of grapefruit juice [151]. The use of kava (Piper methysticum Forst. F.) has been associated with severe hepatotoxicity. This adverse effect was not previously encountered with the traditional beverage, which was prepared as a water infusion in contrast to the commercial products which are extracted with organic solvents. Kavalactones, the active principles in kava, are potent inhibitors of several of the CYP 450 enzymes, suggesting a high potential for causing pharmacokinetic interactions with drugs and other herbs which are metabolized by the same CYP 450 enzymes [152].
Furthermore, some kavalactones have been shown to possess pharmacological effects, such as blockade of GABA receptors and sodium and calcium ion channels, which may lead to pharmacodynamic interactions with other substances that possess similar pharmacological proprieties. St. John’s wort (Hypericum perforatum L.), used extensively for the treatment of mild-to-moderate clinical depression, has long been considered safer than the conventional pharmaceutical agents. However, its ability, through its active constituents hypericin, pseudohypericin, and hyperforin, to induce intestinal P-gp/MRD1 and both intestinal and hepatic CYP3A4 enzyme could markedly reduce the distribution and disposition of their co-substrates. Besides, St. John’s wort is a potent uptake inhibitor of the neurotransmitters serotonin, noradrenaline, and dopamine, all of which have a role in mood control. However, presently there is very little evidence to substantiate actual pharmacokinetic and/or pharmacodynamic interaction between drugs and St. John’s wort [153]. Arold et al. also report no relevant interaction with alprazolam, caffeine, tolbutamide, and digoxin by treatment with a low-hyperforin St John’s wort extract [154]. However, coadministration of imatinib with St. John’s wort may compromise imatinib’s clinical efficacy [155]. Against the background of proven efficacy in mild-to-moderate depressive disorders and an excellent tolerability profile in monotherapy, there is sufficient evidence from the interaction studies and case reports suggesting that St John’s wort may induce the cytochrome P450 (CYP) 3A4 enzyme system and the P-gp drug transporter in a clinically relevant manner, thereby reducing the efficacy of co-medications. Drugs most prominently affected and contraindicated for concomitant use with St John’s wort are metabolized via both CYP3A4 and P-gp pathways, including HIV protease inhibitors, HIV non-nucleoside reverse transcriptase inhibitors (only CYP3A4), the immunosuppressants ciclosporin and tacrolimus, and the antineoplastic agents' irinotecan and imatinib mesylate. Efficacy of hormonal contraceptives may be impaired as reflected by case reports of irregular bleedings and unwanted pregnancies. The St John’s wort constituent hyperforin is probably responsible for CYP3A4 induction via activation of a nuclear steroid/pregnane and xenobiotic receptor (SXR/PXR) and hypericin may be assumed to be the P-gp inducing compound, although the available evidence is less convincing. Thus, combinations of St John’s wort with serotonergic agents and other antidepressants should be restricted due to potential central pharmacodynamic interactions [156].
Expert opinion
The biology of cancer is much better understood today than it was a few decades ago. Despite this increasing knowledge, the incidence of cancer is higher today than it was 30 years ago. Epidemiology has revealed that certain diseases are more common among people of some cultures than others. Cancers of the lung, colon, prostate, and breast are prevalent in Western countries; they are not as commonplace in Eastern countries. Similarly, diseases of the head and neck and the cervix are most common in India, whereas stomach cancer is most prevalent in Japan. Migration from native to the adopted country, however, exposes an individual to the same cancer risk and incidence as that of others living in the chosen country. This phenomenon suggests a minimal role for genotype and a more significant part of a lifestyle. These findings have prompted the US National Cancer Institute to examine the traditional concepts of lifestyle that play a role in cancer prevention. Ayurveda is an intricate system of healing that originated in India thousands of years ago. Historical evidence of Ayurveda can be found in the ancient books of wisdom known as the Vedas that were written over 6000 years ago. Ayurveda provides novel approaches to cancer prevention that are considered safe. Ayurvedic treatment of cancer involves prevention, surgical removal of tumors, herbal remedies, dietary modifications, and spiritual therapies (e.g., detoxification, rejuvenation, prayer, music therapy, aromatherapy, gem therapy, sound therapy, stress relief, meditation, yoga, and astrology). The current emphasis should be laid on the identification of the mechanism of action of ayurvedic drugs and the prevention and treatment of cancer by combining these treatments with modern developments in medicine.
Conclusions
It is estimated that > 80% of the world’s population cannot afford modern medicines. In addition to cost, current cancer therapies are minimally effective and exhibit toxicities that are intolerable in most cases. This review presents evidence that agents derived from plants used in Ayurvedic medicine can be used not only to prevent cancer but also to treat disease. Because of their pharmacological safety, these agents can be used alone or as adjuncts to current chemotherapeutic agents to enhance therapeutic effects and minimize chemotherapy-induced toxicity. Because cancer is primarily a disease of older age, finding less toxic therapies is a major priority. This review reveals that the molecular targets of chemopreventive agents are similar to those currently being used to treat cancer. Tumor cells use multiple cell survival pathways to prevail, and agents that can suppress these multiple pathways have great potential in the treatment of cancer. The evidence indicates that most of the plant-based agents used in Ayurvedic medicine do, indeed, suppress multiple pathways. More research is needed for these agents to reach their full therapeutic potential.
Acknowledgments
The authors would like to thank for ChaRhonda Chilton for a careful review of the manuscript. This work was supported by the Clayton Foundation for Research, a Department of Defense US Army Breast Cancer Research Programme grant (BC010610), a PO1 grant (CA91844) from the National Institutes of Health on lung chemoprevention, and a P50 Head and Neck SPORE grant from the National Institutes of Health (all to BB Aggarwal).
References & Bibliography
1. NEWMAN DJ, CRAGG GM,
SNADER KM: Natural products as sources
of new drugs over the period 1981-2002.
J. Nat. Prod. (2003) 66:1022-1037.
•• This review describes the influence of
natural product structures in modern
drug designs.
2. CRAIG WJ: Phytochemicals: guardians
of our health. J. Am. Diet. Assoc. (1997)
97:S199-S204.
3. CRAIG WJ: Health-promoting properties
of common herbs. Am. J. Clin. Nutr. (1999)
70:491S-499S.
4. COUSSENS LM, WERB Z: Inflammation
and cancer. Nature (2002) 420:860-867.
•• This review expanded the concept that
inflammation is a critical component
of tumour progression.
5. BALKWILL F, MANTOVANI A:
Inflammation and cancer: back to Virchow?
Lancet (2001) 357:539-545.
6. HAHN WC, WEINBERG RA:
Rules for making human tumor cells.
N. Engl. J. Med. (2002) 347:1593-1603.
7. AGGARWAL BB: Nuclear factor-kappaB:
the enemy within. Cancer Cell (2004)
6:203-208.
•• This article reviews the role of NF-κB
signalling in cancer.
8. EFERL R, WAGNER EF:
AP-1: a double-edged sword in
tumorigenesis. Nat. Rev. Cancer
(2003) 3:859-868.
9. GARG A, AGGARWAL BB: Nuclear
transcription factor-kappaB as a target for
cancer drug development. Leukemia (2002)
16:1053-1068.
10. KORUTLA L, CHEUNG YJ,
MENDELSOHN J, KUMAR R:
Inhibition of ligand-induced activation
of epidermal growth factor receptor
tyrosine phosphorylation by curcumin.
Carcinogenesis (1995) 16:1741-1745.
11. HONG RL, SPOHN WH, HUNG MC:
Curcumin inhibits tyrosine kinase activity
of p185neu and also depletes p185neu.
Clin. Cancer Res. (1999) 5:1884-1891.
12. YU H, JOVE R: The STATs of cancer-new
molecular targets come of age.
Nat. Rev. Cancer (2004) 4:97-105.
13. HONG J, LAMBERT JD, LEE SH et al.:
Involvement of multidrug resistance-associated proteins in regulating cellular
levels of (-)-epigallocatechin-3-gallate and
its methyl metabolites. Biochem. Biophys.
Res. Commun. (2003) 310:222-227.
14. LO MUZIO L, STAIBANO S,
PANNONE G et al.: The human
multidrug resistance gene (MDR-1):
immunocytochemical detection
of its expression in oral SCC.
Anti-Cancer Res. (2000) 20:2891-2897.
15. JAIN V, DAS SN, LUTHRA K et al.:
Differential expression of multidrug
resistance gene product, P-glycoprotein,
in normal, dysplastic and malignant oral
mucosa in India. Int. J. Cancer (1997)
74:128-133.
16. KIM WJ, KAKEHI Y, WU WJ et al.:
Expression of multidrug resistance-related
genes (mdrl, MRP, GST-pi and DNA
topoisomerase II) in urothelial cancers.
Br. J. Urol. (1996) 78:361-368.
17. SUBBARAMAIAH K,
DANNENBERG AJ: Cyclooxygenase 2:
a molecular target for cancer prevention and
treatment. Trends Pharmacol. Sci. (2003)
24:96-102.
18. FOLKMAN J: Fundamental concepts of
the angiogenic process. Curr. Mol. Med.
(2003) 3:643-651.
19. HENDRIX MJ, SEFTOR EA, HESS AR,
SEFTOR RE: Vasculogenic mimicry
and tumour-cell plasticity: lessons from
melanoma. Nat. Rev. Cancer (2003)
3:411-421.
20. SHERR CJ: The Pezcoller lecture: cancer
cell cycles revisited. Cancer Res. (2000)
60:3689-3695.
21. CHARAKA: Charaka Samhita.
Chaukhamba Orientalia. Varanasi,
India (700 BC).
•• The Charaka Samhita is believed to have
arisen around 400-200 BC. It is felt to be
one of the oldest and the most important
ancient authoritative writings on
Ayurveda.
22. SUSRUTA: Susruta Samhita. Chaukhamba
Surbharati Publications. Varanasi, India
(700 BC).
•• The Sushruta Samhita presents the field of
Ayurvedic surgery (shalya). This branch of
medicine arose in part from the exigencies
of dealing with the effects of war
23. MISRA B: Bhawa Prakash Nighantu.
Chaukhamba Publications. Varanasi,
India (1600 AD).
24. KAPOOR LD: Handbook of ayurvedic
medicinal plants. CRC Press. Florida (1990).
25. BALACHANDRAN P,
GOVINDARAJAN R: Cancer-an
ayurvedic perspective. Pharmacol. Res.
(2005) 51:19-30.
26. SINGH RH: An assessment of the
ayurvedic concept of cancer and a new
paradigm of anticancer treatment in
Ayurveda. J. Altern. Complement. Med.
(2002) 8:609-614.
27. SMIT HF, WOERDENBAG HJ,
SINGH RH et al.: Ayurvedic herbal
drugs with possible cytostatic activity.
J. Ethnopharmacol. (1995) 47:75-84.
28. URIZAR NL, MOORE DD:
GUGULIPID: A natural cholesterollowering agent. Ann. Rev. Nutr. (2003)
23:303-313.
29. SINAL CJ, GONZALEZ FJ:
Guggulsterone: an old approach to a new
problem. Trends Endocrinol. Metab. (2002)
13:275-276.
30. GUJRAL ML, SAREEN K, TANGRI KK
et al.: Antiarthritic and anti-inflammatory
activity of gum guggul (Balsamodendron
mukul Hook). Indian J. Physiol. Pharmacol.
(1960) 4:267-273.
31. SHARMA JN: Comparison of the antiinflammatory activity of Commiphora
Mukul (an indigenous drug) with those
of phenylbutazone and ibuprofen in
experimental arthritis induced
by mycobacterial adjuvant.
Arzneimittelforschung (1977) 27:1455-1457.
32. SINGH BB, MISHRA LC,
VINJAMURY SP et al.: The effectiveness
of Commiphora mukul for osteoarthritis
of the knee: an outcomes study.
Altern. Ther. Health Med. (2003) 9:74-79.
33. URIZAR NL, LIVERMAN AB,
DODDS DT et al.: A natural product that
lowers cholesterol as an antagonist ligand
for FXR. Science (2002) 296:1703-1706.
34. WU J, XIA C, MEIER J et al.:
The hypolipidemic natural product
guggulsterone acts as an antagonist of the
bile acid receptor. Mol. Endocrinol. (2002)
16:1590-1597.
35. CUI J, HUANG L, ZHAO A et al.:
Guggulsterone is a farnesoid X receptor
antagonist in coactivator association assays
but acts to enhance transcription of bile
salt export pump. J. Biol. Chem. (2003)
278:10214-10220.
36. MESELHY MR: Inhibition of LPS-induced
NO production by the oleogum resin of
Commiphora wightii and its constituents.
Phytochemistry (2003) 62:213-218.
37. YAMAMOTO Y, GAYNOR RB:
Therapeutic potential of inhibition of the
NF-kappaB pathway in the treatment of
inflammation and cancer. J. Clin. Invest.
(2001) 107:135-142.
38. SHISHODIA S, AGGARWAL BB:
Guggulsterone inhibits NF-kappaB and
IkappaBalpha kinase activation, suppresses
expression of anti-apoptotic gene products,
and enhances apoptosis. J. Biol. Chem.
(2004) 279:47148-47158.
39. BHARTI AC, DONATO N,
AGGARWAL BB: Curcumin
(diferuloylmethane) inhibits constitutive
and IL-6-inducible STAT3 phosphorylation
in human multiple myeloma cells.
J. Immunol. (2003) 171:3863-3871.
40. ANTO RJ, MUKHOPADHYAY A,
DENNING K, AGGARWAL BB:
Curcumin (diferuloylmethane) induces
apoptosis through activation of caspase-8,
BID cleavage and cytochrome c release: its
suppression by ectopic expression of Bcl-2
and Bcl-xl. Carcinogenesis (2002)
23:143-150.
41. MUKHOPADHYAY A,
BUESO-RAMOS C, CHATTERJEE D
et al.: Curcumin downregulates cell survival
mechanisms in human prostate cancer cell
lines. Oncogene (2001) 20:7597-7609.
42. MUKHOPADHYAY A, BANERJEE S,
STAFFORD LJ et al.: Curcumin-induced
suppression of cell proliferation correlates
with down-regulation of cyclin D1
expression and CDK4-mediated
retinoblastoma protein phosphorylation.
Oncogene (2002) 21:8852-8861.
43. AGGARWAL BB, KUMAR A,
BHARTI AC: Anticancer potential of
curcumin: preclinical and clinical studies.
Anti-Cancer Res. (2003) 23:363-398.
44. SHISHODIA S, POTDAR P,
GAIROLA CG, AGGARWAL BB:
Curcumin (diferuloylmethane) downregulates cigarette smoke-induced NFkappaB activation through inhibition
of IkappaBalpha kinase in human lung
epithelial cells: correlation with suppression
of COX-2, MMP-9 and cyclin D1.
Carcinogenesis (2003) 24:1269-1279.
45. BHARTI AC, TAKADA Y,
AGGARWAL B: Curcumin
(diferuloylmethane) inhibits receptor
activator of NF-kappa B ligand-induced
NF-kappa B activation in osteoclast
precursors and suppresses
osteoclastogenesis.
J. Immunol. (2004) 172:5940-5947.
46. BHARTI AC, SHISHODIA S,
REUBEN JM et al.: Nuclear factor-kappaB
and STAT3 are constitutively active in
CD138+ cells derived from multiple
myeloma patients, and suppression of these
transcription factors leads to apoptosis.
Blood (2004) 103:3175-3184.
47. BHARTI AC, DONATO N, SINGH S,
AGGARWAL BB: Curcumin
(diferuloylmethane) down-regulates the
constitutive activation of nuclear factorkappa B and IkappaBalpha kinase in human
multiple myeloma cells, leading to
suppression of proliferation and induction
of apoptosis. Blood (2003) 101:1053-1062.
48. AGGARWAL S, TAKADA Y, SINGH S
et al.: Inhibition of growth and survival of
human head and neck squamous cell
carcinoma cells by curcumin via modulation
of nuclear factor-kappaB signaling.
Int. J. Cancer (2004) 111:679-692.
49. AGGARWAL BB, TAKADA Y,
OOMMEN OV: From chemoprevention
to chemotherapy: common targets and
common goals. Expert Opin. Investig. Drugs
(2004) 13:1327-1338.
50. LI L, AGGARWAL BB, SHISHODIA S
et al.: Nuclear factor-kappaB and IkappaB
kinase are constitutively active in human
pancreatic cells, and their down-regulation
by curcumin (diferuloylmethane) is
associated with the suppression of
proliferation and the induction of apoptosis.
Cancer (2004) 101:2351-2362.
51. DORAI T, AGGARWAL BB: Role of
chemopreventive agents in cancer therapy.
Cancer Lett. (2004) 215:129-140.
52. TAKADA Y, BHARDWAJ A, POTDAR P,
AGGARWAL BB: Nonsteroidal antiinflammatory agents differ in their ability to
suppress NF-kappaB activation, inhibition
of expression of cyclooxygenase-2 and
cyclin D1, and abrogation of tumor
cell proliferation. Oncogene (2004)
23:9247-9258.
53. AGGARWAL BB, SHISHODIA S:
Suppression of the nuclear factor-kappaB
activation pathway by spice-derived
phytochemicals: reasoning for seasoning.
Ann. NY Acad. Sci. (2004) 1030:434-441.
54. BHARTI AC, TAKADA Y,
AGGARWAL BB: PARP cleavage and
caspase activity to assess chemosensitivity.
Methods Mol. Med. (2005) 111:69-78.
55. SIWAK DR, SHISHODIA S,
AGGARWAL BB, KURZROCK R:
Curcumin-induced antiproliferative and
proapoptotic effects in melanoma cells
are associated with suppression of IkappaB
kinase and nuclear factor kappaB activity
and are independent of the B-Raf/mitogenactivated/extracellular signal-regulated
protein kinase pathway and the Akt
pathway. Cancer (2005) 104:879-890.
56. SHISHODIA S, AMIN HM, LAI R,
AGGARWAL BB: Curcumin
(diferuloylmethane) inhibits constitutive
NF-kappaB activation, induces G1/S arrest,
suppresses proliferation, and induces
apoptosis in mantle cell lymphoma.
Biochem. Pharmacol. (2005) 70:700-713.
57. YAN C, JAMALUDDIN MS,
AGGARWAL B et al.: Gene expression
profiling identifies activating transcription
factor 3 as a novel contributor to the
proapoptotic effect of curcumin.
Mol. Cancer Ther. (2005) 4:233-241.
58. SHISHODIA S, GETHI G,
AGGARWAL BB: Curcumin: Getting back
to the roots. Ann. N. Y. Acad. Sci. (2005)
(In Press).
59. AGGARWAL BB, KUMAR A,
BHARTI AC: Therapeutic potential of
curcumin derived from turmeric (Curcuma
longa). Marcel Dekker. New York (2004).
60. AGGARWAL BB, KUMER S,
AGGARWAL S, SHISHODIA S:
Curcumin derived from turmeric
(Curcuma longa): A spice for all seasons.
In: Phytochmeicals in Cancer
Chemoprevention, Bagchi D,
Preuss HG (Eds.), CRC press (2005).
•• This review describes the therapeutic
potential of curcumin.
61. PAUL B, MASIH I, DEOPUJARI J,
CHARPENTIER C: Occurrence of
resveratrol and pterostilbene in age-old
darakchasava, an ayurvedic medicine from
India. J. Ethnopharmacol. (1999) 68:71-76.
62. MANNA SK, MUKHOPADHYAY A,
AGGARWAL BB: Resveratrol suppresses
TNF-induced activation of nuclear
transcription factors NF-kappa B, activator
protein-1, and apoptosis: potential role
of reactive oxygen intermediates and lipid
peroxidation. J. Immunol. (2000)
164:6509-6519.
63. BANERJEE S, BUESO-RAMOS C,
AGGARWAL BB: Suppression of 7,12-
dimethylbenz(a)anthracene-induced
mammary carcinogenesis in rats by
resveratrol: role of nuclear factor-kappaB,
cyclooxygenase 2, and matrix
metalloprotease 9. Cancer Res. (2002)
62:4945-4954.
64. ESTROV Z, SHISHODIA S, FADERL S
et al.: Resveratrol blocks interleukin-1betainduced activation of the nuclear
transcription factor NF-kappaB, inhibits
proliferation, causes S-phase arrest,
and induces apoptosis of acute myeloid
leukemia cells. Blood (2003) 102:987-995.
65. AGGARWAL BB, BHARDWAJ A,
AGGARWAL RS et al.: Role of resveratrol
in prevention and therapy of cancer:
preclinical and clinical studies.
Anti-Cancer Res. (2004) 24:2783-2840.
66. CARLSON BA, DUBAY MM,
SAUSVILLE EA et al.: Flavopiridol
induces G1 arrest with inhibition of cyclindependent kinase (CDK) 2 and CDK4 in
human breast carcinoma cells. Cancer Res.
(1996) 56:2973-2978.
67. LOSIEWICZ MD, CARLSON BA,
KAUR G et al.: Potent inhibition of CDC2
kinase activity by the flavonoid L86-8275.
Biochem. Biophys. Res. Commun. (1994)
201:589-595.
68. DE AZEVEDO WF Jr,
MUELLER-DIECKMANN HJ,
SCHULZE-GAHMEN U et al.:
Structural basis for specificity and potency
of a flavonoid inhibitor of human CDK2,
a cell cycle kinase. Proc. Natl. Acad.
Sci. USA (1996) 93:2735-2740.
69. WORLAND PJ, KAUR G,
STETLER-STEVENSON M et al.:
Alteration of the phosphorylation state of
p34cdc2 kinase by the flavone L86-8275
in breast carcinoma cells. Correlation with
decreased H1 kinase activity. Biochem.
Pharmacol. (1993) 46:1831-1840.
70. KAUR G, STETLER-STEVENSON M,
SEBERS S et al.: Growth inhibition with
reversible cell cycle arrest of carcinoma cells
by flavone L86-8275. J. Natl. Cancer Inst.
(1992) 84:1736-1740.
71. BIBLE KC, KAUFMANN SH:
Flavopiridol: a cytotoxic flavone that
induces cell death in noncycling A549
human lung carcinoma cells.
Cancer Res. (1996) 56:4856-4861.
72. KONIG A, SCHWARTZ GK,
MOHAMMAD RM et al.: The
novel cyclin-dependent kinase inhibitor
flavopiridol downregulates Bcl-2 and
induces growth arrest and apoptosis
in chronic B-cell leukemia lines.
Blood (1997) 90:4307-4312.
73. ARGUELLO F, ALEXANDER M,
STERRY JA et al.: Flavopiridol induces
apoptosis of normal lymphoid cells,
causes immunosuppression, and has potent
antitumor activity In vivo against human
leukemia and lymphoma xenografts.
Blood (1998) 91:2482-2490.
74. BYRD JC, SHINN C, WASELENKO JK
et al.: Flavopiridol induces apoptosis in
chronic lymphocytic leukemia cells via
activation of caspase-3 without evidence
of bcl-2 modulation or dependence on
functional p53. Blood (1998) 92:3804-3816.
75. GOJO I, ZHANG B, FENTON RG:
The cyclin-dependent kinase inhibitor
flavopiridol induces apoptosis in multiple
myeloma cells through transcriptional
repression and down-regulation of Mcl-1.
Clin. Cancer Res. (2002) 8:3527-3538.
76. PATEL V, SENDEROWICZ AM,
PINTO D Jr et al.: Flavopiridol, a novel
cyclin-dependent kinase inhibitor,
suppresses the growth of head and neck
squamous cell carcinomas by inducing
apoptosis. J. Clin. Invest. (1998)
102:1674-1681.
77. SHAPIRO GI, SUPKO JG,
PATTERSON A et al.: A Phase II trial
of the cyclin-dependent kinase inhibitor
flavopiridol in patients with previously
untreated stage IV non-small cell lung cancer.
Clin. Cancer Res. (2001) 7:1590-1599.
78. SENDEROWICZ AM, SAUSVILLE EA:
Preclinical and clinical development of
cyclin-dependent kinase modulators.
J. Natl. Cancer Inst. (2000) 92:376-387.
79. KARP JE, ROSS DD, YANG W et al.:
Timed sequential therapy of acute leukemia
with flavopiridol: in vitro model for a
Phase I clinical trial. Clin. Cancer Res.
(2003) 9:307-315.
80. BIBLE KC, KAUFMANN SH: Cytotoxic
synergy between flavopiridol (NSC 649890,
L86-8275) and various antineoplastic
agents: the importance of sequence of
administration. Cancer Res. (1997)
57:3375-3380.
81. NAHTA R, IGLEHART JD,
KEMPKES B, SCHMIDT EV:
Rate-limiting effects of Cyclin D1 in
transformation by ErbB2 predicts synergy
between herceptin and flavopiridol.
Cancer Res. (2002) 62:2267-2271.
82. WU K, WANG C, D’AMICO M et al.:
Flavopiridol and trastuzumab synergistically
inhibit proliferation of breast cancer cells:
association with selective cooperative
inhibition of cyclin D1-dependent
kinase and Akt signaling pathways.
Mol. Cancer Ther. (2002) 1:695-706.
83. CARTEE L, MAGGIO SC, SMITH R
et al.: Protein kinase C-dependent
activation of the tumor necrosis factor
receptor-mediated extrinsic cell death
pathway underlies enhanced apoptosis in
human myeloid leukemia cells exposed
to bryostatin 1 and flavopiridol.
Mol. Cancer Ther. (2003) 2:83-93.
84. KIM DM, KOO SY, JEON K et al.:
Rapid induction of apoptosis by
combination of flavopiridol and tumor
necrosis factor (TNF)-alpha or TNF-related
apoptosis-inducing ligand in human cancer
cell lines. Cancer Res. (2003) 63:621-626.
85. TAKADA Y, AGGARWAL BB:
Flavopiridol inhibits NF-kappaB activation
induced by various carcinogens and
inflammatory agents through inhibition
of IkappaBalpha kinase and p65
phosphorylation: abrogation of
cyclin D1, cyclooxygenase-2, and matrix
metalloprotease-9. J. Biol. Chem. (2004)
279:4750-4759.
86. KITAYAMA T, OKAMOTO T, HILL RK
et al.: Chemistry of zerumbone. 1.
Simplified isolation, conjugate addition
reactions, and a unique ring contracting
transannular reaction of its dibromide.
J. Org. Chem. (1999) 64:2667-2672.
87. DEV S: Zermbone, a monocytclic
sesquiterpene ketone. Chem. & Ind.
(1956):1051.
88. MURAKAMI A, TAKAHASHI M,
JIWAJINDA S et al.: Identification of
zerumbone in Zingiber zerumbet
Smith as a potent inhibitor of 12-Otetradecanoylphorbol-13-acetate-induced
Epstein-Barr virus activation. Biosci.
Biotechnol. Biochem. (1999) 63:1811-1812
89. MURAKAMI A, TAKAHASHI D,
KOSHIMIZU K, OHIGASHI H:
Synergistic suppression of superoxide and
nitric oxide generation from inflammatory
cells by combined food factors.
Mutat. Res. (2003) 523-524:151-161.
90. TANAKA T, SHIMIZU M, KOHNO H
et al.: Chemoprevention of azoxymethaneinduced rat aberrant crypt foci by dietary
zerumbone isolated from Zingiber
zerumbet. Life Sci. (2001) 69:1935-1945.
91. MURAKAMI A, TANAKA T, LEE JY
et al.: Zerumbone, a sesquiterpene in
subtropical ginger, suppresses skin tumor
initiation and promotion stages in ICR
mice. Int. J. Cancer (2004) 110:481-490.
92. OZAKI Y, KAWAHARA N, HARADA M:
Anti-inflammatory effect of Zingiber
cassumunar Roxb and its active principles.
Chem. Pharm. Bull. (Tokyo) (1991)
39:2353-2356.
93. KIRANA C, MCINTOSH GH,
RECORD IR, JONES GP: Antitumor
activity of extract of Zingiber aromaticum
and its bioactive sesquiterpenoid zerumbone.
Nutr. Cancer (2003) 45:218-225.
94. MURAKAMI A, TAKAHASHI D,
KINOSHITA T et al.: Zerumbone, a
Southeast Asian ginger sesquiterpene,
markedly suppresses free radical generation,
proinflammatory protein production, and
cancer cell proliferation accompanied by
apoptosis: the alpha,beta-unsaturated
carbonyl group is a prerequisite.
Carcinogenesis (2002) 23:795-802.
95. MURAKAMI A, HAYASHI R,
TANAKA T et al.: Suppression of dextran
sodium sulfate-induced colitis in mice
by zerumbone, a subtropical ginger
sesquiterpene, and nimesulide: separately
and in combination. Biochem. Pharmacol.
(2003) 66:1253-1261.
96. MURAKAMI A, MATSUMOTO K,
KOSHIMIZU K, OHIGASHI H: Effects of
selected food factors with chemopreventive
properties on combined lipopolysaccharideand interferon-gamma-induced IkappaB
degradation in RAW264.7 macrophages.
Cancer Lett. (2003) 195:17-25.
97. TAKADA Y, MURAKAMI A,
AGGARWAL BB: Zerumbone abolishes
NF-kappaB and IkappaBalpha kinase
activation leading to suppression of
antiapoptotic and metastatic gene
expression, upregulation of apoptosis,
and downregulation of invasion.
Oncogene (2005) 24:6957-6969
98. KIRTIKAR KR, BASU BD:
Indian Medicinal Plants (1935).
99. NO AUTHORS LISTED: Hager’s
handbuch der Pharmazeut, Praxis,
Springer-Verlag. Berlin (1972).
100. NO AUTHORS LISTED: The Wealth
of India; Raw Materials, CSIR publications,
Delhi (1948).
101. REDDY GK, CHANDRAKASAN G,
DHAR SC: Studies on the metabolism of
glycosaminoglycans under the influence
of new herbal anti-inflammatory agents.
Biochem. Pharmacol. (1989) 38:3527-3534.
102. SAFAYHI H, MACK T, SABIERAJ J et al.:
Boswellic acids: novel, specific, nonredox
inhibitors of 5-lipoxygenase. J. Pharmacol.
Exp. Ther. (1992) 261:1143-1146.
103. SAFAYHI H, SAILER ER, AMMON HP:
Mechanism of 5-lipoxygenase inhibition
by acetyl-11-keto-beta-boswellic acid.
Mol. Pharmacol. (1995) 47:1212-1216.
104. AMMON HP, SAFAYHI H, MACK T,
SABIERAJ J: Mechanism of
antiinflammatory actions of curcumine
and boswellic acids. J. Ethnopharmacol.
(1993) 38:113-119.
105. KAPIL A, MOZA N: Anticomplementary
activity of boswellic acids-an inhibitor of
C3-convertase of the classical complement
pathway. Int. J. Immunopharmacol. (1992)
14:1139-1143.
106. SAFAYHI H, RALL B, SAILER ER,
AMMON HP: Inhibition by boswellic
acids of human leukocyte elastase. J.
Pharmacol. Exp. Ther. (1997) 281:460-463.
107. GERHARDT H, SEIFERT F, BUVARI P
et al.: [Therapy of active Crohn disease
with Boswellia serrata extract H 15].
Z. Gastroenterol. (2001) 39:11-17.
108. GUPTA I, PARIHAR A, MALHOTRA P
et al.Effects of Boswellia serrata gum resin in
patients with ulcerative colitis. Eur. J. Med.
Res. (1997) 2:37-43.
109. KRIEGLSTEIN CF, ANTHONI C,
RIJCKEN EJ et al.: Acetyl-11-keto-betaboswellic acid, a constituent of a herbal
medicine from Boswellia serrata resin,
attenuates experimental ileitis.
Int. J. Colorectal Dis. (2001) 16:88-95.
110. REDDY GK, DHAR SC: Effect of a new
non-steroidal anti-inflammatory agent
on lysosomal stability in adjuvant induced
arthritis. Ital. J. Biochem. (1987) 36:205-217.
111. SHARMA ML, BANI S, SINGH GB:
Anti-arthritic activity of boswellic acids
in bovine serum albumin (BSA)-induced
arthritis. Int. J. Immunopharmacol. (1989)
11:647-652.
112. SAFAYHI H, MACK T, AMMON HP:
Protection by boswellic acids against
galactosamine/endotoxin-induced hepatitis
in mice. Biochem. Pharmacol. (1991)
41:1536-1537.
113. KIMMATKAR N, THAWANI V,
HINGORANI L, KHIYANI R:
Efficacy and tolerability of Boswellia serrata
extract in treatment of osteoarthritis of
knee-a randomized double blind placebo
controlled trial. Phytomedicine (2003) 10:3-7.
114. WINKING M, SARIKAYA S,
RAHMANIAN A et al.: Boswellic acids
inhibit glioma growth: a new treatment
option? J. Neurooncol. (2000) 46:97-103.
115. GLASER T, WINTER S, GROSCURTH P
et al.: Boswellic acids and malignant glioma:
induction of apoptosis but no modulation
of drug sensitivity. Br. J. Cancer (1999)
80:756-765.
116. JING Y, NAKAJO S, XIA L et al.:
Boswellic acid acetate induces
differentiation and apoptosis in leukemia
cell lines. Leuk. Res. (1999) 23:43-50.
117. SHAO YC, HO T, CHIN CK et al.:
Inhibitory activity of boswellic acids from
Boswellia serrata against human leukemia
HL-60 cells in culture. Planta Med. (1998)
64:328-331.
118. LIU JJ, NILSSON A, OREDSSON S et al.:
Boswellic acids trigger apoptosis via a
pathway dependent on caspase-8 activation
but independent on Fas/Fas ligand
interaction in colon cancer HT-29 cells.
Carcinogenesis (2002) 23:2087-2093.
119. ZHAO W, ENTSCHLADEN F, LIU H
et al.: Boswellic acid acetate induces
differentiation and apoptosis in highly
metastatic melanoma and fibrosarcoma
cells. Cancer Detect. Prev. (2003) 27:67-75.
120. HUANG MT, BADMAEV V, DING Y
et al.: Anti-tumor and anti-carcinogenic
activities of triterpenoid, beta-boswellic
acid. Biofactors (2000) 13:225-230.
121. STEINMETZ KA, POTTER JD:
Vegetables, fruit, and cancer prevention:
a review. J. Am. Diet. Assoc. (1996)
96:1027-1039.
122. TAKADA Y, ANDREEFF M,
AGGARWAL BB: Indole-3-carbinol
suppresses NF-kappaB and IkappaBalpha
kinase activation, causing inhibition of
expression of NF-kappaB-regulated
antiapoptotic and metastatic gene products
and enhancement of apoptosis in myeloid and
leukemia cells. Blood (2005) 106:641-649.
123. AGGARWAL BB, ICHIKAWA H:
Molecular targets and anticancer potential
of indole-3-carbinol and its derivatives.
Cell Cycle (2005) 4:1201-1215.
124. GARG AK, BUCHHLOZ TA,
AGGARWAL BB: Chemosensitization
and radiosensitization of tumors by plant
polyphenols. Antioxidant and Redox
Signaling (2005) (In Press).
125. DEVI PU, RAO BS, SOLOMON FE:
Effect of plumbagin on the radiation
induced cytogenetic and cell cycle changes
in mouse Ehrlich ascites carcinoma in vivo.
Indian J. Exp. Biol. (1998) 36:891-895.
126. SHARADA AC, SOLOMON FE,
DEVI PU et al.: Antitumor and
radiosensitizing effects of withaferin A
on mouse Ehrlich ascites carcinoma in vivo.
Acta Oncol. (1996) 35:95-100.
127. GANASOUNDARI A, ZARE SM,
DEVI PU: Modification of bone marrow
radiosensensitivity by medicinal plant
extracts. Br. J. Radiol. (1997) 70:599-602.
128. CHENDIL D, RANGA RS,
MEIGOONI D et al.: Curcumin confers
radiosensitizing effect in prostate cancer cell
line PC-3. Oncogene (2004) 23:1599-1607.
129. JAGETIA GC, NAYAK V,
VIDYASAGAR MS: Evaluation of
the antineoplastic activity of guduchi
(Tinospora cordifolia) in cultured HeLa
cells. Cancer Lett. (1998) 127:71-82.
130. JAGETIA GC, VENKATESHA VA:
Effect of mangiferin on radiation-induced
micronucleus formation in cultured human
peripheral blood lymphocytes. Environ.
Mol. Mutagen. (2005) 46:12-21.
131. JAGETIA GC, BALIGA MS, MALAGI KJ,
SETHUKUMAR KAMATH M: The
evaluation of the radioprotective effect of
Triphala (an ayurvedic rejuvenating drug)
in the mice exposed to gamma-radiation.
Phytomedicine (2002) 9:99-108.
132. JAGETIA GC, BALIGA MS:
Treatment with Alstonia scholaris enhances
radiosensitivity in vitro and in vivo. Cancer
Biother. Radiopharm. (2003) 18:917-929
133. YOUNT G, QIAN Y, MOORE D et al.:
Berberine sensitizes human glioma cells,
but not normal glial cells, to ionizing
radiation in vitro. J. Exp. Ther. Oncol.
(2004) 4:137-143.
134. SAMAILA DB, TOY J, WANG RC,
ELEGBEDE JA: Monoterpenes enhanced
the sensitivity of head and neck cancer
cells to radiation treatment in vitro.
Anti-Cancer Res. (2004) 24:3089-3095.
135. HORSMAN MR, SIEMANN DW,
CHAPLIN DJ, OVERGAARD J:
Nicotinamide as a radiosensitizer in
tumours and normal tissues: the importance
of drug dose and timing. Radiother. Oncol.
(1997) 45:167-174.
136. HARBOTTLE A, DALY AK,
ATHERTON K, CAMPBELL FC: Role of
glutathione S-transferase P1, P-glycoprotein
and multidrug resistance-associated protein
1 in acquired doxorubicin resistance.
Int. J. Cancer (2001) 92:777-783.
137. IERSEL ML, PLOEMEN JP, STRUIK I
et al.: Inhibition of glutathione S-transferase
activity in human melanoma cells by
alpha,beta-unsaturated carbonyl derivatives.
Effects of acrolein, cinnamaldehyde, citral,
crotonaldehyde, curcumin, ethacrynic acid,
and trans-2-hexenal. Chem. Biol. Interact.
(1996) 102:117-132.
138. DUVOIX A, MORCEAU F,
DELHALLE S et al.: Induction of apoptosis
by curcumin: mediation by glutathione
S-transferase P1-1 inhibition. Biochem.
Pharmacol. (2003) 66:1475-1483.
139. OGISO Y, TOMIDA A, LEI S et al.:
Proteasome inhibition circumvents solid
tumor resistance to topoisomerase IIdirected drugs. Cancer Res. (2000)
60:2429-2434.
140. JANA NR, DIKSHIT P, GOSWAMI A,
NUKINA N: Inhibition of proteasomal
function by curcumin induces apoptosis
through mitochondrial pathway.
J. Biol. Chem. (2004) 279:11680-11685.
141. FULDA S, DEBATIN KM: Sensitization
for tumor necrosis factor-related apoptosisinducing ligand-induced apoptosis by the
chemopreventive agent resveratrol.
Cancer Res. (2004) 64:337-346
142. DEVI PU: Withania somnifera Dunal
(Ashwagandha): potential plant source of
a promising drug for cancer chemotherapy
and radiosensitization. Indian J. Exp. Biol.
(1996) 34:927-932.
143. SINGH I, SHARMA A, NUNIA V,
GOYAL PK: Radioprotection of Swiss
albino mice by Emblica officinalis.
Phytother. Res. (2005) 19:444-446.
144. PRASAD NS, RAGHAVENDRA R,
LOKESH BR, NAIDU KA: Spice phenolics
inhibit human PMNL 5-lipoxygenase.
Prostaglandins Leukot. Essent. Fatty Acids
(2004) 70:521-528.
145. ZHOU S, LIM LY, CHOWBAY B:
Herbal modulation of P-glycoprotein.
Drug Metab. Rev. (2004) 36:57-104.
146. SHOBA G, JOY D, JOSEPH T et al.:
Influence of piperine on the
pharmacokinetics of curcumin in animals
and human volunteers. Planta Med. (1998)
64:353-356.
•• The study shows that piperine enhances
the serum concentration, extent of
absorption and bioavailability of curcumin
in both rats and humans with no adverse
effects.
147. LAMBERT JD, HONG J, KIM DH et al.:
Piperine enhances the bioavailability of the
tea polyphenol (-)-epigallocatechin-3-gallate
in mice. J. Nutr. (2004) 134:1948-1952.
148. PALUMBO G, BACCHI S, PALUMBO P
et al.: [Grapefruit juice: potential drug
interaction]. Clin. Ter. (2005) 156:97-103.
149. LE GOFF-KLEIN N, KLEIN L,
HERIN M et al.: Inhibition of in-vitro
simvastatin metabolism in rat liver
microsomes by bergamottin, a component
of grapefruit juice. J. Pharm. Pharmacol.
(2004) 56:1007-1014.
150. LILJA JJ, NEUVONEN M,
NEUVONEN PJ: Effects of regular
consumption of grapefruit juice on
the pharmacokinetics of simvastatin.
Br. J. Clin. Pharmacol. (2004) 58:56-60.
151. LILJA JJ, KIVISTO KT, NEUVONEN PJ:
Duration of effect of grapefruit juice on the
pharmacokinetics of the CYP3A4 substrate
simvastatin. Clin. Pharmacol. Ther. (2000)
68:384-390.
Affiliation
152. ANKE J, RAMZAN I: Pharmacokinetic
and pharmacodynamic drug interactions
with Kava (Piper methysticum Forst. f.).
J. Ethnopharmacol. (2004) 93:153-160.
153. SINGH YN: Potential for interaction
of kava and St. John’s wort with drugs.
J. Ethnopharmacol. (2005) 100:108-113.
154. AROLD G, DONATH F, MAURER A
et al.: No relevant interaction with
alprazolam, caffeine, tolbutamide, and
digoxin by treatment with a low-hyperforin
St John’s wort extract. Planta Med. (2005)
71:331-337.
155. SMITH P, BULLOCK JM, BOOKER BM
et al.: The influence of St. John’s wort on
the pharmacokinetics and protein binding
of imatinib mesylate. Pharmacotherapy
(2004) 24:1508-1514.
156. MANNEL M: Drug interactions with
St John’s wort : mechanisms and clinical
implications. Drug Saf. (2004) 27:773-797.
157. KHAN S, BALICK MJ: Therapeutic plants
of Ayurveda: a review of selected clinical
and other studies for 166 species. J. Altern.
Complement. Med. (2001) 7:405-515.
158. SAXENA S, PANT N, JAIN DC,
BHAKUNI RS: Antimalarial agents
from plant sources. Curr. Sci. (2003)
85:1314-1329.
159. THOMAS SC: Medicinal plants: Culture,
utilization and phytopharmacology,
Techno Publishing Co., Inc., USA (2000).
160. ROTBLATT M, ZIMENT I: Evidence
based herbal medicine, Hanley & Belfus, Inc.
(2001).
161. MILLER LG, MURRAY WJ:
Herbal medicine: A clinician’s guide,
Pharmaceuticals Products Press, NY (1998).
162. NO AUTHORS LISTED: PDR For Herbal
medicines, Medical Economics Company,
Montvale, New Jersey (1999).
163. TEWARI L: Traditional Himalayan
Medicine System and its Materia Medica,
HIST, India (2001).
Website
Affiliation
Affiliation
201. http://www.clinicaltrials.gov
A service of the US National Institute
of Health (2005)
Affiliation
Affiliation
Affiliation
Bharat B Aggarwal†1 PhD,
Haruyo Ichikawa2 PhD,
Prachi Garodia3 MD, Priya Weerasinghe4 PhD,
Gautam Sethi2 PhD, Indra D Bhatt2 PhD,
Manoj K Pandey2 PhD, Shishir Shishodia5 PhD
& Muraleedharan G Nair6 PhD
†Author for correspondence
1.Ransom Horne, Jr., Professor of Cancer
Research, Professor of Cancer Medicine
(Biochemistry), and Chief, Cytokine Research
Laboratory, The University of Texas, MD
Anderson Cancer Center, Cytokine Research
Laboratory, Department of Experimental
Therapeutics, Box 143, 1515 Holcombe
Boulevard, Houston, Texas 77030, USA
Tel: +1 713 792 3503/6459;
Fax: +1 713 794 1613;
E-mail: aggarwal@mdanderson.org
2.Postdoctoral Fellow, The University of Texas,
MD Anderson Cancer Center, Cytokine Research
Laboratory, Department of Experimental
Therapeutics, Box 143, 1515 Holcombe
Boulevard, Houston, Texas 77030, USA
3.Doctor, The University of Texas, MD Anderson
Cancer Center, Cytokine Research Laboratory,
Department of Experimental Therapeutics,
Box 143, 1515 Holcombe Boulevard,
Houston, Texas 77030, USA
4.Instructor, The University of Texas, MD
Anderson Cancer Center, Cytokine Research
Laboratory, Department of Experimental
Therapeutics, Box 143, 1515 Holcombe
Boulevard, Houston, Texas 77030, USA
5.Assistant Professor, Texas Southern University,
Department of Biology, 3100 Cleburne Street,
Houston, Texas 77004, USA
6.Professor, Michigan State University,
173 National Food Safety and Toxicology Center,
East Lansing, MI 48824, USA
Copyright © 2023 Farmers For Health Global Initiative - All Rights Reserved.
Powered by GoDaddy